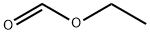| Identification | Back Directory | [Name]
RARECHEM AL BI 0212 | [CAS]
19064-14-3 | [Synonyms]
RARECHEM AL BI 0212
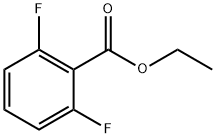
ETHYL 2,6-DIFLUOROBENZOATE
Ethyl 2,6-difluorobenzoate 97%
2,6-Difluorobenzoic acid, ethyl ester
Benzoic acid, 2,6-difluoro-, ethyl ester | [EINECS(EC#)]
943-621-0 | [Molecular Formula]
C9H8F2O2 | [MDL Number]
MFCD06204338 | [MOL File]
19064-14-3.mol | [Molecular Weight]
186.16 |
| Hazard Information | Back Directory | [Synthesis]
At -78 °C, n-butyllithium (n-BuLi, 1.67 M hexane solution, 1.32 mL, 2.2 mmol) was slowly added dropwise to a solution of tetrahydrofuran (THF, 3 mL) of 1,3-difluorobenzene (383 mg, 2.0 mmol), and the dropwise process was continued for 30 min. Subsequently, ethyl formate (1.6 mL, 20 mmol) was added to the reaction mixture and stirring was continued at -78 °C. After maintaining this temperature for 3 hours, iodine (I2, 1523 mg, 6 mmol), potassium carbonate (K2CO3, 1382 mg, 10 mmol), and ethanol (EtOH, 3 mL) were added to the reaction system, and then the mixture was warmed to room temperature and stirred for 14 hours. After completion of the reaction, the reaction was quenched with saturated aqueous sodium sulfite (Na2SO3) (5 mL) and extracted with chloroform (CHCl3, 3 x 20 mL). The organic layers were combined, washed with brine and dried over anhydrous sodium sulfate (Na2SO4) to give ethyl 2,6-difluorobenzoate in 77% yield. If necessary, the product can be purified by short column chromatography (silica gel (SiO2) as stationary phase, eluent is petroleum ether: ethyl acetate = 9:1) to obtain pure ethyl 2,6-difluorobenzoate in the form of colorless oil. | [References]
[1] Tetrahedron, 2012, vol. 68, # 24, p. 4701 - 4709 |
|
|