| Identification | Back Directory | [Name]
1-(4-IODO-PHENYL)-PIPERIDIN-2-ONE | [CAS]
385425-15-0 | [Synonyms]
Apixaban II
1-(4-Iodophenyl)-2-piperidinone
2-Piperidinone,1-(4-iodophenyl)-
1-(4-IODO-PHENYL)-PIPERIDIN-2-ONE | [Molecular Formula]
C11H12INO | [MDL Number]
MFCD04037128 | [MOL File]
385425-15-0.mol | [Molecular Weight]
301.12 |
| Chemical Properties | Back Directory | [Boiling point ]
446.1±28.0 °C(Predicted) | [density ]
1.670 | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [pka]
-0.43±0.20(Predicted) | [Appearance]
White to off-white Solid | [InChI]
InChI=1S/C11H12INO/c12-9-4-6-10(7-5-9)13-8-2-1-3-11(13)14/h4-7H,1-3,8H2 | [InChIKey]
OYDSTJMVOOOYDW-UHFFFAOYSA-N | [SMILES]
N1(C2=CC=C(I)C=C2)CCCCC1=O |
| Hazard Information | Back Directory | [Synthesis]
General steps:
1. triethylamine (31.10 g, 307.38 mmol) was added to a solution of 4-iodoaniline (30.0 g, 136.98 mmol) in tetrahydrofuran (800 mL) under nitrogen protection.
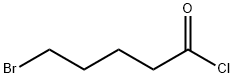
2. The reaction mixture was cooled to 0 °C, then a solution of 5-bromovaleryl chloride (32.7 g, 163.90 mmol) in tetrahydrofuran (200 mL) was slowly added dropwise over 30 min.
3. After the dropwise addition was completed, the reaction mixture was brought to room temperature and stirred for 16 hours.
4. After the reaction was complete, the mixture was cooled to 0 °C again and potassium tert-butoxide (46.0 g, 410 mmol) was slowly added.
5. The reaction mixture was brought to room temperature and stirred for 18 hours. 6.
6. After completion of the reaction, the solvent was removed by concentration under reduced pressure to give a thick oily substance.
7. The pH was adjusted to 2.0 with 3N hydrochloric acid solution and extracted with ethyl acetate (3 x 500 mL).
8. The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product.
9. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, 0%-100%) to afford 1-(4-iodophenyl)piperidin-2-one (20.0 g, yield 48.5%) as an off-white solid.
Characterization data:
Melting point: 108-110°C.
1H NMR (400 MHz, CDCl3) δ: 1.93-1.95 (m, 4H), 2.55 (t, J = 6.2 Hz, 2H), 3.62 (t, J = 5.2 Hz, 2H), 7.02 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.4 Hz, 2H).
IR (KBr) υ: 3256, 3049, 2936, 2864, 1634, 1576, 1482, 1434, 1164, 1000, 819, 709 cm-1.
MS: 302 (M + 1). | [References]
[1] Journal of Medicinal Chemistry, 2007, vol. 50, # 22, p. 5339 - 5356
[2] Patent: US2003/232804, 2003, A1. Location in patent: Page 94
[3] Patent: US2017/50964, 2017, A1. Location in patent: Paragraph 0699
[4] Patent: WO2010/30983, 2010, A2. Location in patent: Page/Page column 28 |
|
|