
(+/-)1-(1-Naphthyl)ethylamine synthesis
- Product Name:(+/-)1-(1-Naphthyl)ethylamine
- CAS Number:42882-31-5
- Molecular formula:C12H13N
- Molecular Weight:171.24
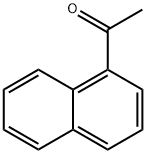
941-98-0

42882-31-5
General procedure for the synthesis of 1-naphthylethylamine from 1-naphthylethanone: The corresponding ketone (10 mmol, specifically 1.14 g of 1a, 0.98 g of 1b, 1.48 g of 1c, 1.46 g of 1d, 1.46 g of 1e, 1.32 g of 1f, 1.20 g of 1g, 1.70 g of 1h, 1.70 g of 1i) was mixed with hydroxylamine hydrochloride ( 15 mmol, 1.04 g), ammonium formate (60 mmol, 3.78 g) and zinc powder (30 mmol, 1.96 g) in methanol (30 mL) and the reaction was stirred under reflux conditions. After completion of the reaction, the reaction mixture was filtered through Celite. The filtrate was subjected to vacuum rotary evaporation to remove the solvent. The residue was treated with concentrated hydrochloric acid followed by the addition of 4 mL of HCl solution and 30 mL of water and extracted with ether (2 x 20 mL) to remove organic impurities. The aqueous phase was adjusted to pH=10 with ammonia and extracted with dichloromethane (4 x 25 mL). The organic phases were combined, washed with saturated saline, dried over anhydrous sodium sulfate, and finally the solvent was removed in vacuum to obtain the target product 1-naphthylethylamine.
![Phosphinic amide, N-[1-(1-naphthalenyl)ethyl]-P,P-diphenyl-](/CAS/20210305/GIF/344248-49-3.gif)
344248-49-3
0 suppliers
inquiry

42882-31-5
235 suppliers
$13.00/1g
Yield:42882-31-5 80%
Reaction Conditions:
with hydrogenchloride in ethanol at 20; for 2 h;
Steps:
17 EXAMPLE 17; Synthesis of 1-Naphthylethylamine
Gaseous hydrogen chloride was bubbled through a stirred solution of N-diphenylphosphinyl-1-naphthylethylamine in ethyl alcohol for 2 hours at room temperature. The reaction mixture was concentrated, made basic by the addition of an aqueous solution of sodium hydroxide (2 M) and extracted with dichloromethane (3×10 ml). The organic layer was washed with brine (1×10 ml), dried over magnesium sulphate and concentrated to give the product as a yellow liquid in 80% yield.
References:
US6696608,2004,B1 Location in patent:Page column 28

941-98-0
379 suppliers
$5.00/10g

42882-31-5
235 suppliers
$13.00/1g

3886-70-2
510 suppliers
$5.00/1g

42882-31-5
235 suppliers
$13.00/1g

3886-70-2
510 suppliers
$5.00/1g
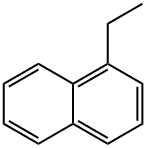
1127-76-0
140 suppliers
$6.00/1g

42882-31-5
235 suppliers
$13.00/1g
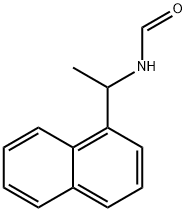
49681-33-6
30 suppliers
inquiry

42882-31-5
235 suppliers
$13.00/1g