
1,2,4-Triazolylsodium synthesis
- Product Name:1,2,4-Triazolylsodium
- CAS Number:41253-21-8
- Molecular formula:C2H4N3Na
- Molecular Weight:93.06

288-88-0

41253-21-8
General procedure for the synthesis of sodium 1,2,4-triazole from 1,2,4-triazole: To a three-necked flask equipped with a mechanical stirrer, an internal thermometer, and a reflux condenser, 200 mL of anhydrous methanol and 45 mL of a 30% methanol solution of sodium methanol (containing 0.25 mol of sodium methanol) were added, under dry nitrogen protection. At room temperature, 17.4 g (0.25 mol) of 1,2,4-triazole was added in batches. After completion of addition, the reaction mixture was heated to reflux temperature and stirred for 4 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. To the remaining oily residue was added 200 mL of dichloromethane and stirred at room temperature for 15 minutes. The precipitated solid product was collected by filtration to afford 22.5 g of sodium 1,2,4-triazolate (yield 98% of the theoretical value) as a colorless powder. The purity of the product was confirmed by 1H-NMR spectroscopy and no residue of the raw material 1,2,4-triazole was detected.

288-88-0
827 suppliers
$10.00/5g

41253-21-8
293 suppliers
$5.00/5g
Yield:41253-21-8 98%
Reaction Conditions:
with sodium methylate in methanol; for 4 h;Heating / reflux;
Steps:
Catalyst 1: Sodium 1,2,4-triazolate
[0061] Catalyst 1: Sodium 1,2,4-triazolate [0062] A three-necked-flask stirring apparatus with mechanical stirrer, internal thermometer and reflux condenser was charged under dry nitrogen with 200 ml of dry methanol and 45 ml of a 30% strength methanolic solution of sodium methoxide, corresponding to 0.25 mol of sodium methoxide. 17.4 g (0.25 mol) of 1,2,4-triazole was added thereto in portions at room temperature. After the end of addition of the 1,2,4-triazole the reaction mixture was stirred at reflux temperature for 4 h. The solvent was subsequently distilled off under reduced pressure and the oily residue which remained was admixed at room temperature with 200 ml of methylene chloride. The mixture was stirred at room temperature for 15 min and the precipitated solid product was filtered off. This gave 22.5 g of sodium 1,2-4-triazolate (yield: 98% of theory) in the form of a colourless powder. The product was pure according to its 1H-NMR spectrum and free of the 1,2,4-triazole used.
References:
US2004/49028,2004,A1 Location in patent:Page 5

288-88-0
827 suppliers
$10.00/5g
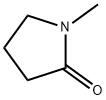
872-50-4
986 suppliers
$10.00/100ML

41253-21-8
293 suppliers
$5.00/5g
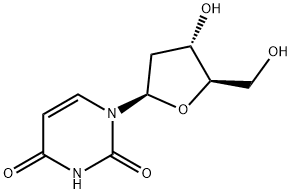
951-78-0
500 suppliers
$5.00/100mg
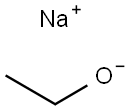
141-52-6
502 suppliers
$10.00/5g

41253-21-8
293 suppliers
$5.00/5g

66656-44-8
1 suppliers
inquiry

141-52-6
502 suppliers
$10.00/5g

80991-41-9
8 suppliers
inquiry

41253-21-8
293 suppliers
$5.00/5g

59495-22-6
4 suppliers
inquiry