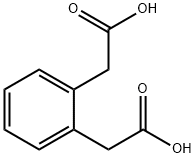
1,2-Phenylenediacetic acid synthesis
- Product Name:1,2-Phenylenediacetic acid
- CAS Number:7500-53-0
- Molecular formula:C10H10O4
- Molecular Weight:194.18

613-73-0

7500-53-0
The general procedure for the synthesis of 2,2'-(1,2-phenylene)diacetic acid from 1,2-phenylenediacetonitrile was as follows: 1,2-phenylenediacetonitrile (5.0 g) was dissolved in 50 mL of concentrated hydrochloric acid and heated and refluxed for 3 hours. After the reaction was completed, 30 mL of water was added and heating was continued overnight. The reaction mixture was cooled to room temperature and washed with ether. Subsequently, the organic layer was extracted twice with aqueous sodium carbonate. The aqueous layers were combined, acidified to pH 2-3 with hydrochloric acid and extracted with ether. The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated to give 2,2'-(1,2-phenylene)diacetic acid as a light yellow solid in 56% yield with a melting point of 123-125 °C. The product was confirmed by 1H NMR (250 MHz, DMSO-d6): δ 3.58 (s, 4H), 7.20 (s, 4H), 12.34 (br s, 2H); 13C NMR (63 MHz, DMSO-d6): δ 37.1 (2C), 126.8 (2C), 130.6 (2C), 134.1 (2C), 172.4 (2C).

613-73-0
139 suppliers
$22.80/5g

7500-53-0
211 suppliers
$27.00/5g
Yield: 56%
Reaction Conditions:
with hydrogenchloride;waterReflux;
Steps:
3
Example No.3. Synthesis of 2,2'-(l,2-phenylene)diacetic acid.[0055] The above dinitrile was dissolved in 50 mL concentrated HC1 and refluxed for 3 h. Water (30 mL) was added and the reaction heated overnight, then cooled and washed with ether. The organic layer was extracted twice with sodium carbonate. Combined aqueous layers were acidified and extracted with ether, which was dried (MgSO4), filtered and concentrated. The diacid was obtained as a pale yellow solid in 56% yield: mp 123 - 125 °C; 1H NMR (250 MHz, DMSO-D6) 5 3.58 (s, 4H), 7.20 (s, 4H), 12.34 (br s, 2H); 13C NMR (63 MHz, DMSO-D6) 37.1 (2C), 126.8 (2C), 130.6 (2C), 134.1 (2C), 172.4 (2C).
References:
EXXONMOBIL RESEARCH AND ENGINEERING COMPANY;DAKKA, Jihad M.;MOZELESKI, Edmund J.;BAUGH, Lisa S.;BENITEZ, Frank, M. WO2011/41349, 2011, A1 Location in patent:Page/Page column 19-20

1559-81-5
7 suppliers
$500.51/5MG

7500-53-0
211 suppliers
$27.00/5g

529-34-0
486 suppliers
$6.00/10g
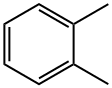
95-47-6
616 suppliers
$10.00/25g

7500-53-0
211 suppliers
$27.00/5g

91-13-4
253 suppliers
$14.00/1g

7500-53-0
211 suppliers
$27.00/5g
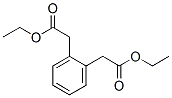
17532-66-0
1 suppliers
inquiry

7500-53-0
211 suppliers
$27.00/5g