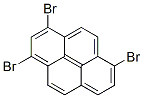
1,3,6-tribromopyrene synthesis
- Product Name:1,3,6-tribromopyrene
- CAS Number:38303-36-5
- Molecular formula:C16H7Br3
- Molecular Weight:438.9388
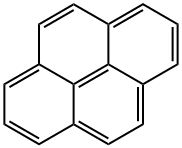
129-00-0
344 suppliers
$9.00/1g

38303-36-5
6 suppliers
inquiry
Yield:38303-36-5 92.3%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide;
Steps:
P.6 [Production of tribromopyrene]
(Production Example 6) [Production of triphenylpyrene] [Production of tribromopyrene] A 1000 mL four-necked flask was fitted with a dropping funnel, a reflux condenser tube, a three-way cock and a thermometer, and was purged with nitrogen. The flask was then charged with 20.0 g of pyrene (reagent made by Aldrich Co.) and 200 ml of DMF, and then was again purged with nitrogen. The system was heated at an internal temperature of 70°C to dissolve pyrene. Into 400 ml of DMF was dissolved 108.9 g of NBS (reagent made by Tokyo Kasei Kogyo Co., Ltd.), and this was dropwise added thereto from the dropping funnel over 20 minutes. After the end of the addition, the heating temperature was raised from 70°C to 130°C. At this temperature, reaction was conducted for 8 hours. The resultant was cooled, and then the resultant solid was collected by suction filtration. This was washed with ethanol to yield 39.3 g of a crude product of 1,3,6-tribromopyrene (yield: 92.3%). The resultant crude product was recrystallized from o-dichlorobenzene having a volume 15 times as much as the volume of the sample to collect light gray needle crystals. From LC analysis, the purity of the collected product was 91% (as other components, dibromopyrene and tetrabromopyrene were found).
References:
EP1818322,2007,A1

1714-29-0
307 suppliers
$6.00/1g

38303-36-5
6 suppliers
inquiry

1714-29-0
307 suppliers
$6.00/1g

27973-29-1
278 suppliers
$6.00/250mg

38303-35-4
94 suppliers
$40.00/100mg

38303-36-5
6 suppliers
inquiry

129-00-0
344 suppliers
$9.00/1g

27973-29-1
278 suppliers
$6.00/250mg

38303-35-4
94 suppliers
$40.00/100mg

38303-36-5
6 suppliers
inquiry