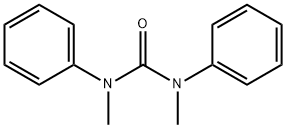
1,3-Dimethyl-1,3-diphenylurea synthesis
- Product Name: 1,3-Dimethyl-1,3-diphenylurea
- CAS Number:611-92-7
- Molecular formula:C15H16N2O
- Molecular Weight:240.3

4285-42-1
54 suppliers
$100.00/5g

611-92-7
109 suppliers
$40.00/250mg
Yield:611-92-7 95%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium hydrogencarbonate in 1,4-dioxane at 100; for 48 h;Catalytic behavior;Schlenk technique;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;
Steps:
2. General Procedures for the Synthesis of Urea Derivatives
General procedure: A 10 mL Schlenk tube equipped with a magnetic stirring bar was charged with arylcarbamoyl chlorides (0.2 mmol, 1.0 equiv), and KHCO3 (40 mg, 0.4 mmol), and then Pd(PPh3)4 (0.01 mmol, 11.56 mg) were added. Finally, 1,4-dioxane (1.0 mL) was added to the mixture via syringe at room temperature under N2. The tube was sealed and put into a preheated oil bath a 100 °C for 48 h. The mixture was cooled to room temperature, quenched with water (5 mL), and diluted with ethyl acetate (5 mL). The layers were separated, and the aqueous layer was extracted with 2 × 5 mL of ethyl acetate. The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The crude product was then purified by flash chromatography on silica gel (H), eluting with 30-35% ethylacetate/petroleum ether. 3. Spectral Data of Urea Derivatives 1,3-Dimethyl-1,3-diphenylurea (2a)3 Yield, 95% (22.8 mg); white solid, mp 120-122 °C .1H NMR (500 MHz, CDCl3) δ 7.04 (t, J = 7.8 Hz, 4H), 6.93 (t, J = 7.4 Hz, 2H), 6.79 (d, J = 7.5 Hz, 4H), 3.19 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 160.3, 144.6, 127.6, 124.7, 123.8, 38.3.
References:
Fan, Aihong;Peng, Jinsong;Zhou, Dun;Li, Xiang;Chen, Chunxia [Synthetic Communications,2020,p. 1 - 12] Location in patent:supporting information

77-78-1
322 suppliers
$22.00/25g

102-07-8
306 suppliers
$6.00/25g

611-92-7
109 suppliers
$40.00/250mg
201230-82-2
1 suppliers
inquiry
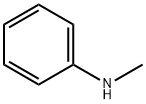
100-61-8
400 suppliers
$8.00/1g

611-92-7
109 suppliers
$40.00/250mg

93-61-8
295 suppliers
$10.00/5G

124-38-9
133 suppliers
$214.00/14L

100-61-8
400 suppliers
$8.00/1g

611-92-7
109 suppliers
$40.00/250mg
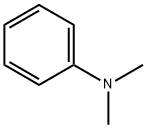
121-69-7
567 suppliers
$10.00/25mL

93-61-8
295 suppliers
$10.00/5G

102-07-8
306 suppliers
$6.00/25g

74-88-4
357 suppliers
$15.00/10g

611-92-7
109 suppliers
$40.00/250mg