
1,8-Diaminooctane synthesis
- Product Name:1,8-Diaminooctane
- CAS Number:373-44-4
- Molecular formula:C8H20N2
- Molecular Weight:144.26

629-40-3

373-44-4
The general procedure for the synthesis of 1,8-octanediamine from 1,6-dicyanohexane was as follows: a 500 mL autoclave was assembled with a thermocouple thermometer, a stirrer (two-piece tilting paddle), a pressure gauge, and a hydrogen purge line. To the autoclave were added 175 g of 1,6-dicyanohexane, 86.0 g of methanol, 2.0 g of 28% sodium methacrylate-methanol solution (0.64% w/w), and 10.5 g of nickel catalyst (based on nickel monomers, which accounted for 6.0 w/w% of 1,6-dicyanohexane). The water content of the solution was 920 ppm at this point, as measured by a Karl Fischer hydrometer (manufactured by Hiranuma Sangyo Co., Ltd.) Upon completion of the dosing, hydrogen substitution was performed, and then the pressure was increased to 5 MPa. The temperature was raised to an internal temperature of 90 °C, and the ramping process lasted for approximately 1 hour. Hydrogen consumption was observed from about 70°C. The reaction was started at 90°C and hydrogen consumption essentially stopped after about 6 hours. After continuing the reaction for 2 hours, the reaction was cooled to room temperature, depressurized, and the reaction solution was filtered to remove the catalyst and the filtrate was washed with 100 g of methanol. The total weight of the 1,8-diaminooctane methanol reaction solution obtained was 377.4 g. The area of 1,8-diaminooctane was analyzed by GC to be 94.3% and the reaction yield was 96.7%.

629-41-4
377 suppliers
$9.00/10g

373-44-4
240 suppliers
$6.00/5g
Yield:373-44-4 78%
Reaction Conditions:
with ammonia;carbonylchloro[4,5-bis(diisopropylphosphinomethyl)acridine]hydridoruthenium(II) in tert-Amyl alcohol at 140; for 48 h;Product distribution / selectivity;Autoclave;
Steps:
3
Example 3; Reaction of 1,8-octanediol; The reaction was carried out under the same conditions as in Example 1 using 10.0 mmol of 1,8-octanediol. Yield and conversion were determined by gas chromatography using commercially available reference compounds. Yield of linear primary diamine: 78%, conversion of the linear diol: >99%.; Example 1; Reaction of 1,19-nonadecanediol; 3.01 g (10.0 mmol) of 1,19-nonadecanediol and 151 mg (0.25 mol %) of carbonylchloro[4,5-bis(diisopropylphosphinomethyl)acridine]hydridoruthenium(II) were dissolved under protective gas in 25 ml of 2-methyl-2-butanol and transferred to an autoclave provided with stirrer, heating and temperature measuring facility. 6 ml of liquid ammonia were subsequently introduced into the autoclave by means of a spindle press. The autoclave was closed and the contents stirred at 140° C. for 48 hours. This resulted in an increase in the internal pressure from 22 to 40 bar. After cooling, the contents of the reactor were filtered through kieselguhr, the filtrate was evaporated to dryness and the residue subjected to a bulb tube distillation. Yield of linear primary diamine: 2.02 g (68% of theory; bp0.8 mbar=170-185° C.), conversion of the linear diol: >99%.
References:
Evonik Degussa GmbH US2012/203033, 2012, A1 Location in patent:Page/Page column 4

629-40-3
153 suppliers
$28.10/25g

373-44-4
240 suppliers
$6.00/5g

1740-54-1
16 suppliers
inquiry

373-44-4
240 suppliers
$6.00/5g
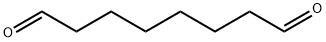
638-54-0
13 suppliers
inquiry

373-44-4
240 suppliers
$6.00/5g

111-19-3
178 suppliers
$14.00/5g

373-44-4
240 suppliers
$6.00/5g