
1-BROMO-4-FLUORO-2-NITROBENZENE synthesis
- Product Name:1-BROMO-4-FLUORO-2-NITROBENZENE
- CAS Number:446-09-3
- Molecular formula:C6H3BrFNO2
- Molecular Weight:220

394-01-4

446-09-3
General procedure for the synthesis of 2-bromo-5-fluoronitrobenzene from 2-nitro-4-fluorobenzoic acid: 6.2 mg of silver sulfate, 36.3 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-o-phenanthroline, 37 mg of 2-nitro-4-fluorobenzoic acid, and 30.9 mg of sodium bromide were sequentially added to a Silak reactor tube fitted with a magnetic stirrer, followed by 4 mL of dimethyl sulfoxide as solvent. The reaction mixture was heated to 160°C under an oxygen atmosphere with continuous stirring for 24 hours. Upon completion of the reaction, distilled water was added to the reaction mixture to quench the reaction, followed by extraction with ethyl acetate three times at 10 mL each. the organic phases were combined, and 21.1 mg of 2-bromo-5-fluoronitrobenzene was obtained after concentration in 48% yield.
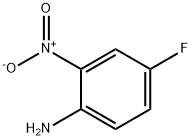
364-78-3
286 suppliers
$7.00/5g

446-09-3
245 suppliers
$7.00/5g
Yield:446-09-3 79.5%
Reaction Conditions:
Stage #1:2-nitro-4-fluoroaniline with sulfuric acid;sodium nitrite in water at 10 - 20;Large scale;
Stage #2: with hydrogen bromide;copper(I) bromide in water at 40 - 45;Large scale;
Steps:
1.3; 2.3 Bromine reaction on diazo
Add 1100 g of 2-nitro-4-fluoroaniline to the reaction vessel, add about 30% of sulfuric acid 12000 g, stir, then add a solution of 600 g of sodium nitrite and 2000 mL of water, and add dropwise. The temperature is controlled at 10 to 20 ° C. After the addition is completed, the mixture is stirred for a period of time to prepare a diazonium salt for use.Add 240 g of cuprous bromide and 1800 g of hydrobromic acid to another reaction flask.Stir and control the temperature range of 40 ~ 45 °C to add the above diazonium salt, add, continue to stir,Keep the reaction for a period of time, let cool, add dichloroethane, stir, separate the liquid, and steam,1225 g of 2-nitro-4-fluorobromobenzene was obtained as a brown solid, the content was 98%, and the yield was 79.5%
References:
Changzhou University;Chen Xingquan;Dong Yanmin CN109553534, 2019, A Location in patent:Paragraph 0014; 0016; 0017; 0021; 0023; 0024

448-39-5
67 suppliers
$27.00/1g

446-09-3
245 suppliers
$7.00/5g

394-01-4
255 suppliers
$8.00/5g

446-09-3
245 suppliers
$7.00/5g

402-67-5
275 suppliers
$6.00/5g

446-09-3
245 suppliers
$7.00/5g
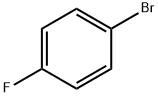
460-00-4
633 suppliers
$6.00/10g

446-09-3
245 suppliers
$7.00/5g