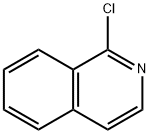
1-Chloroisoquinoline synthesis
- Product Name:1-Chloroisoquinoline
- CAS Number:19493-44-8
- Molecular formula:C9H6ClN
- Molecular Weight:163.6
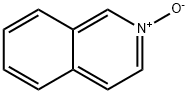
1532-72-5

19493-44-8
The general procedure for the synthesis of 1-chloroisoquinoline from isoquinoline-N-oxide was as follows: phosphoryl chloride (200 mL) was slowly added dropwise to isoquinoline-N-oxide (20.0 g) under ice bath cooling conditions. Subsequently, the reaction mixture was heated to 105 °C and refluxed overnight. Upon completion of the reaction, the excess phosphoryl chloride was removed by distillation under reduced pressure. The residue was quenched with ice water and extracted with dichloromethane. The organic layer was separated, dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography using a mixed solvent of ethyl acetate and petroleum ether as eluent to afford the target product 1-chloroisoquinoline (21.0 g, 85% yield). The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ 8.25-8.31 (m, 2H), 8.08 (d, J = 8.0 Hz, 1H), 7.88-7.91 (m, 2H), 7.80-7.84 (m, 1H). Mass spectrometry (ESI+) showed a molecular ion peak m/z 164.0. HPLC analysis (Method A) showed a retention time (Rt) of 8.29 min and a purity of 96.0%.

1532-72-5
171 suppliers
$15.00/1g

19493-44-8
259 suppliers
$6.00/1g
Yield:19493-44-8 85%
Reaction Conditions:
with trichlorophosphate at 105;Cooling with ice;
Steps:
11.2 Step 2: 1 -chloroisoquinoline
Phosphorus oxychloride (200 ml_) was added dropwise under ice-cold condition to isoquinolin-/V-oxide (20.0 g). The reaction mixture was then heated to reflux at 105 °C overnight. Phosphorus oxychloride was evaporated under reduced pressure, then the residue was quenched with ice and extracted with dichloromethane. The organic layer was separated, dried over sodium sulfate and concentrated. The crude was purified by column chromatography on silica gel using ethylacetate and petroleum ether as eluent (21.0 g; 85%). 1H NMR (400 MHz, DMSO-d6): δ 8.25-8.31 (m, 2H), 8.08 (d, J= 8.0 Hz, 1 H), 7.88-7.91 (m, 2H), 7.80-7.84 (m, 1 H). MS (ESI+): 164.0, HPLC (Method A) Rt 8.29min; HPLC purity 96.0 %
References:
ARES TRADING S.A.;SWINNEN, Dominique;MORANDI, Federica WO2013/92979, 2013, A1 Location in patent:Page/Page column 54

119-65-3
421 suppliers
$5.00/25g

19493-44-8
259 suppliers
$6.00/1g

4594-71-2
55 suppliers
$60.00/100mg

19493-44-8
259 suppliers
$6.00/1g
491-30-5
267 suppliers
$7.00/250mg

19493-44-8
259 suppliers
$6.00/1g

491-30-5
267 suppliers
$7.00/250mg

19493-44-8
259 suppliers
$6.00/1g