
N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide synthesis
- Product Name:N-((R)-1-(naphthalen-1-yl)ethyl)-3-(3-(trifluoroMethyl)phenyl)propanaMide
- CAS Number:1005450-55-4
- Molecular formula:C22H20F3NO
- Molecular Weight:371.4
![2-PropenaMide, N-[(1R)-1-(1-naphthalenyl)ethyl]-3-[3-(trifluoroMethyl)phenyl]-, (2E)-](/CAS/20150408/GIF/1095393-66-0.gif)
1095393-66-0

1005450-55-4
In another synthetic scheme, the reduction of the double bond and amide carbonyl group of (E)-N-((R)-1-naphthalen-1-yl-ethyl)-3-(3-trifluoromethylphenyl)acrylamide is carried out in a stepwise manner. Optionally, either the carbonyl or the olefinic double bond is reduced first. In the case of the reduction of the olefinic double bond, the reaction is realized by hydrogenation in the presence of a metal catalyst. This was done as follows: (E)-N-((R)-1-naphthalen-1-yl-ethyl)-3-(3-trifluoromethylphenyl)acrylamide (1.0 equiv; 0.67 mmol; 250 mg) was suspended with 10 wt% Pd/C (25 mg) in a solvent mixture of methanol and toluene (7 mL/1 mL). The reaction flask was evacuated and backfilled with a hydrogen balloon (repeated three times). The reaction mixture was stirred at room temperature and the progress of the reaction was monitored by high performance liquid chromatography (HPLC).After 90 min, the reaction mixture was filtered through a bed of diatomaceous earth and the solvent in the filtrate was removed under vacuum. The resulting solid was dried in a vacuum oven at 45 °C overnight. The isolated yield was 91.2% (229 mg). The structure of the product was confirmed by 1H NMR and mass spectrometry.1H NMR (400 MHz, CDCl3) δ ppm: 1.6 (d, 3H), 2.39-2.53 (m, 2H), 2.96-3.13 (m, 2H), 5.55 (br.s, 1H), 5.87-5.96 (m, 1H), 7.29-7.38 (m, 2H), 7.39-7.46 (m, 2H), 7.29-7.38 (m, 2H), 7.39-7.38 (m, 2H). 7.39-7.46 (m, 4H), 7.46-7.55 (m, 2H), 7.76-7.82 (m, 1H), 7.83-7.89 (m, 1H), 8.00-8.07 (m, 1H). High Resolution Mass Spectrometry (HRMS) (M+1): 372.1.
![2-PropenaMide, N-[(1R)-1-(1-naphthalenyl)ethyl]-3-[3-(trifluoroMethyl)phenyl]-, (2E)-](/CAS/20150408/GIF/1095393-66-0.gif)
1095393-66-0
9 suppliers
inquiry

1005450-55-4
106 suppliers
$72.00/250mg
Yield: 91.2%
Reaction Conditions:
with hydrogen;palladium on activated carbon in methanol;toluene at 20; for 1.5 h;Product distribution / selectivity;
Steps:
2
hi yet another variation, the reduction of the double bond and amide carbonyl of (E)-N-((i?)-l-naphthalen-l-yl-ethyl)-3-(3-trifluoromethyl-phenyl)-acrylamide is carried out in a stepwise manner. Either the carbonyl group or the alkene double bond can be reduced first. For example, the reduction of the alkene double bond is achieved by hydrogenation in the presence of a metal catalyst.[0123] A suspension of (E)-N-((i?)-l-naphthalen-l-yl-ethyl)-3-(3-trifluoromethyl-phenyl)- acrylamide (1.0 equiv.; 0.67 mmol; 250 mg) and Pd/C 10 wt% (25 mg) was stirred in a mixture of methanol and toluene (7 mL/lmL). The reaction flask was evacuated and back filled using a hydrogen filled balloon (three times). The reaction mixture is stirred at room temperature and the progress of the reaction was monitored using high performance liquid chromatography. After 90 minutes, the reaction mixture was filtered over a bed of celite and the solvent from the filtrate was removed in vacuo. The resulting solid was dried overnight at 450C using a vacuum oven. Isolated yield: 91.2% (229 mg). The identity of the amide was confirmed by 1H NMR and mass spectrometry. (400MHz, CDCl3) δ ppm 1.60 (d, 3H) 2.39- 2.53 (m, 2H) 2.96-3.13 ( m, 2H) 5.55 (br. S., IH) 5.87-5.96 (m, IH) 7.29-7.38 (m, 2H) 7.39- 7.46 (m, 4H) 7.46-7.55 (m, 2H) 7.76-7.82 (m, IH) 7.83-7.89 (m, IH) 8.00-8.07 (m, IH) and HRMS (M+l): 372.1
References:
AMGEN INC. WO2009/2427, 2008, A2 Location in patent:Page/Page column 39

3886-70-2
502 suppliers
$5.00/1g

585-50-2
322 suppliers
$19.00/1g

1005450-55-4
106 suppliers
$72.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1005450-55-4
106 suppliers
$72.00/250mg

3886-70-2
502 suppliers
$5.00/1g
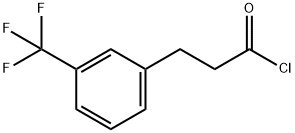
455-03-8
3 suppliers
inquiry

1005450-55-4
106 suppliers
$72.00/250mg

585-50-2
322 suppliers
$19.00/1g
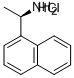
82572-04-1
92 suppliers
$11.00/100mg

1005450-55-4
106 suppliers
$72.00/250mg