1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione synthesis
- Product Name:1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
- CAS Number:1025-15-6
- Molecular formula:C12H15N3O3
- Molecular Weight:249.27

108-80-5

107-18-6

1025-15-6
6.45 g (50 mmol) of cyanuric acid, 670 mg (2.5 mmol) of triphenylphosphine (as an organophosphorus compound) and 25.8 g of xylene (as a solvent) were added to the reaction vessel. Under nitrogen protection, 266 mg of palladium-loaded activated carbon catalyst (trade name E101 NE/W, manufactured by Evonik Degussa Co., Ltd. which is a mixture of zero-valent metallic palladium and divalent palladium compounds with activated carbon in terms of palladium atoms) was added. The mixture was stirred at 95°C for 1 hour. Subsequently, 13.1 g (225 mmol) of propenol (as an allyl-type alcohol) was added slowly and dropwise over 1 h. The reaction lasted for 20 h, during which xylene, propenol, and generated water were removed by azeotropic distillation through a Dean-Stark apparatus. Upon completion of the reaction, the insoluble material was removed by filtration. Analysis of the filtrate showed 98.6% triallyl isocyanurate and 1.4% diallyl isocyanurate in the product based on the conversion of cyanuric acid, and no monoallyl isocyanurate generation was detected.
Yield:1025-15-6 154.6 g
Reaction Conditions:
in N,N-dimethyl-formamide at 120 - 125; for 6 h;
Steps:
1 Synthesis Example 1
Synthesis Example 1 Preparation of Diallylmonoglycidyl Isocyanurate (0207) A reaction vessel was added with 106 g of isocyanuric acid and 420 ml of water of slurry, and then 206 g of 48% sodium hydroxide solution was dropped into the aforementioned mixture. After the mixture was reacted for 2 hours at 60 to 70° C., water was removed, and the mixture was washed by methanol and then dried to obtain 157.5 g of sodium isocyanurate as white crystal. Then, the reaction vessel equipped with a stirrer and a condenser was added with 400 ml of dimethylformamide as a solvent for reacting 157.5 g of sodium isocyanurate and 290.4 g of 3-bromo-1-propylene for 6 hours at 120 to 125° C., and then an inorganic salt was filtered off. Toluene was extracted out and the reactants were washed with water and dried and the solvent was dried to obtain 154.6 g of triallyl isocyanurate (Mw=249.3) as pale brown oil. 8700 ml of dichloromethane was taken as a solvent, and 154.6 g of triallyl isocyanurate and 267 g of m-chloroperbenzoic acid (not higher than 30° C.) was slowly added into a cooler and stirred and reacted at 25° C. for 4 hours. After completing the reaction, 3000 ml of 10% sodium bisulfite solution was slowly added at 20° C., and then an insoluble material was filtered off. Chloroform was added for extraction, and the reactants were washed thoroughly with 10% sodium bisulfite solution and saturated sodium bicarbonate solution. After drying and distilling the solvent, an epoxy compound was obtained. The aforementioned product was purified with silica gel chromatograph to obtain 111.3 g of transparent oil. The obtained epoxy compound was diallylmonoglycidyl isocyanurate (Mw=265).
References:
US9568823,2017,B2 Location in patent:Page/Page column 33

108-80-5
514 suppliers
$6.00/25g

107-18-6
0 suppliers
$24.60/100ml

1025-15-6
347 suppliers
$6.00/10g

4873-09-0
70 suppliers
$75.00/1g
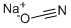
917-61-3
303 suppliers
$24.78/100g

1025-15-6
347 suppliers
$6.00/10g