
tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidine-1-carboxylate
- CAS Number:1048970-17-7
- Molecular formula:C16H30BNO4
- Molecular Weight:311.22
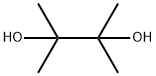
76-09-5
396 suppliers
$8.19/250mg

180695-79-8
194 suppliers
$14.00/1g

274-07-7
170 suppliers
$78.09/5g

1048970-17-7
155 suppliers
$11.00/250mg
Yield:1048970-17-7 36%
Reaction Conditions:
Stage #1:benzo[1,3,2]dioxaborole with bis(cyclopentadienyl)titanium dichloride;potassium carbonate in tert-butyl methyl ether for 0.5 h;
Stage #2:tert-butyl 4-bromo-1-piperidinecarboxylate in tert-butyl methyl ether at 80; under 760.051 Torr; for 24 h;Inert atmosphere;
Stage #3:2,3-dimethyl-2,3-butane diol with triethylamine in tert-butyl methyl ether at 20; for 1 h;Inert atmosphere;
Steps:
80 Example 80
bis(cyclopentadiene)titanium dichloride (denoted as Cp2TiCl2, 0.01mmol, 2.5mg), potassium carbonate (denoted as K2CO3, 0.2mmol, 26.7mg), methyl tert-butyl ether (1mL) Add catecholborane (denoted as HBcat, 0.6mmol, 63μL) into a 38mL pressure tube in sequence, stir for 30min and then add 1-Boc-4-bromopiperidine (denoted as 1ao, 0.2mmol,53mg) in nitrogen Stir at 80°C for 24h under a (1atm) atmosphere, add triethylamine (0.6mmol, 84μL) and pinacol (0.6mmol, 70.8mg) after the reaction, and stir at room temperature for 1h. Then use petroleum ether/ethyl acetate (20:1, v/v) as the eluent to carry out column chromatography purification to obtain the structure compound represented by formula 2ao (colorless and transparent liquid, 1-tert-butyl carboxylate-2-(4) ,4,5,5-Tetramethyl-1,3,2-dioxaborocyclyl)piperidine). The isolated yield is 36%.
References:
CN112645971, 2021, A Location in patent:Paragraph 0229-0231
73183-34-3
587 suppliers
$6.00/5g

180695-79-8
194 suppliers
$14.00/1g

1048970-17-7
155 suppliers
$11.00/250mg

84358-13-4
381 suppliers
$5.00/5g

1048970-17-7
155 suppliers
$11.00/250mg

24424-99-5
865 suppliers
$13.50/25G

181219-01-2
246 suppliers
$13.00/1g

1048970-17-7
155 suppliers
$11.00/250mg

76-09-5
396 suppliers
$8.19/250mg

120347-72-0
17 suppliers
inquiry

24424-99-5
865 suppliers
$13.50/25G

1048970-17-7
155 suppliers
$11.00/250mg