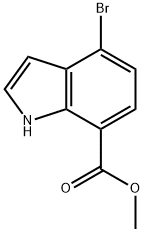
1H-Indole-7-carboxylic acid, 4-broMo-, Methyl ester synthesis
- Product Name:1H-Indole-7-carboxylic acid, 4-broMo-, Methyl ester
- CAS Number:1224724-39-3
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08

1211594-25-0

74-88-4

1224724-39-3
GENERAL STEPS: To a solution of 4-bromo-1H-indole-7-carboxylic acid (33 g, 137 mmol) in N,N-dimethylformamide (DMF, 300 mL) was added cesium carbonate (Cs2CO3, 90 g, 276 mmol) and stirred at room temperature for 1 hour. Subsequently, iodomethane (29.3 g, 206 mmol) was added slowly and dropwise at 0 °C. The reaction mixture was gradually warmed up to room temperature and stirring was continued for 3 hours. After completion of the reaction, the mixture was poured into water and extracted with ethyl acetate (EtOAc, 200 mL x 2). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to afford methyl 4-bromo-1H-indole-7-carboxylate (13.8 g, 20% yield). The structure of the product was confirmed by 1H NMR (CDCl3): δ 9.98 (s, 1H), 7.76-7.74 (d, J = 8 Hz, 1H), 7.39-7.34 (m, 2H), 6.68-6.66 (m, 1H), 4.00 (s, 3H).

1826-67-1
216 suppliers
$51.29/100ml
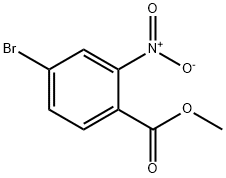
158580-57-5
76 suppliers
$15.00/1g

1224724-39-3
76 suppliers
$65.00/5mg
Yield: 45%
Reaction Conditions:
in tetrahydrofuran;ethanol at -70; for 1 h;Inert atmosphere;
Steps:
10.3 Step 3: Synthesis of compound 10-5
Add compound 10-4 (2.0 g, 7.72 mmol), tetrahydrofuran (20 mL), and replace with nitrogen into a three-necked flask.Cool to -70 in ethanol dry ice bath,Then add ethylmagnesium bromide (28mL, 1mol/L) dropwise,React at this temperature for 1h. The reaction was poured into a saturated ammonium chloride solution, extracted with ethyl acetate three times, the organic phases were combined, and silica gel was added to mix the sample, and then purified by a silica gel column to obtain 680 mg of product 10-5, with a yield of 45%.
References:
CN112608318, 2021, A Location in patent:Paragraph 0233-0234; 0239-0240

1211594-25-0
57 suppliers
$45.00/10mg

74-88-4
358 suppliers
$15.00/10g

1224724-39-3
76 suppliers
$65.00/5mg
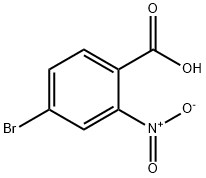
99277-71-1
238 suppliers
$9.00/5g

1224724-39-3
76 suppliers
$65.00/5mg