
2-(4-Fluoro-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetic acid synthesis
- Product Name:2-(4-Fluoro-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetic acid
- CAS Number:1255945-85-7
- Molecular formula:C14H18BFO4
- Molecular Weight:280.1

944317-66-2

1255945-85-7
Step 3: Dissolve 2-fluoro-5-(ethyl formate)phenylboronic acid pinacol ester in a mixture of a solvent miscible with water (e.g., THF, methanol or acetone) and water. Lithium hydroxide (3.0 equiv) was added and the reaction mixture was stirred at room temperature. The progress of the reaction is monitored by TLC and LCMS until the starting material is essentially consumed (reaction time is typically 6-12 hours). Upon completion of the reaction, the THF was concentrated and separated by extraction with ethyl acetate and water. The organic layer was washed with water and the aqueous phases were combined. The washed aqueous phase is acidified with 2N HCl and extracted again with ethyl acetate. The ethyl acetate extracts were combined, dried with sodium sulfate and concentrated in vacuum to give the crude product. The crude product was sufficiently pure in most cases and could be used directly in the next step of the reaction. Reaction conditions: LiOH (3.0 equiv), THF:H2O (1:1), room temperature, 4 h. Yield 65%. Details of the synthesized compounds are given in Table 12.

944317-66-2
45 suppliers
$50.00/50mg

1255945-85-7
35 suppliers
$105.00/100mg
Yield:1255945-85-7 65%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran at 20; for 4 h;
Steps:
7.A.3 Step-3
Step-3 [0392] Boronate ester form step-2 was dissolved in mix of Water and solvents like THF/methanol/Acetone that are miscible in water. To this, lithium hydroxide was added and mixture was stirred at room temperature and monitored by TLC & LCMS till maximum starting was consumed (6-12 hrs required) THF was then concentrated and reaction mass was extracted with ethyl acetate and water. Organic layer was washed with water and combined aq. washings were acidified with 2N HCl and extracted with ethyl acetate. Ethyl acetate extract was dried over sodium sulphate and concentrated in vacuum to get crude product. In most of the cases products were sufficient pure to be used for the next step. The details of compounds synthesized by above method are as below. [0393] The details of compounds synthesized are as below in Table 12. LiOH (3.0 eq.), THF:H2O (1:1), RT, 4 h, 65%.
References:
US2014/194383,2014,A1 Location in patent:Paragraph 0387; 0392-0393
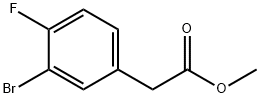
885681-61-8
16 suppliers
inquiry

1255945-85-7
35 suppliers
$105.00/100mg