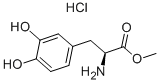
L-3,4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE synthesis
- Product Name:L-3,4-DIHYDROXYPHENYLALANINE METHYL ESTER HYDROCHLORIDE
- CAS Number:1421-65-4
- Molecular formula:C10H14ClNO4
- Molecular Weight:247.68

67-56-1
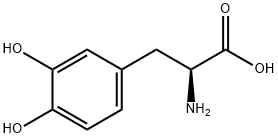
59-92-7

1421-65-4
Methanol (50 mL) was cooled to -5°C in a thermostat bath and thionyl chloride (5 mL, 68.9 mmol) was added slowly and dropwise. Subsequently, 3,4-dihydroxy-L-phenylalanine (10.0 g, 50.7 mmol) was added in batches and the reaction mixture was stirred for 5 min after the addition was completed. The reaction system was gradually warmed up to room temperature and subsequently heated to 50 °C with continuous stirring for 14 hours. Upon completion of the reaction, the reaction solution was concentrated under reduced pressure to afford methyl (S)-2-amino-3-(3,4-dihydroxyphenyl)propionate hydrochloride (14.3 g, 57.7 mmol, quantitative yield), the product was in the form of oil.1H-NMR (400 MHz, CD3OD) δ: 3.04 (dd, 1H, J = 7.4, 14.5 Hz), 3.13 (dd, 1H, J = 5.8, 14.5 Hz), 3.13 (dd, 1H, J = 5.8, 14.5 Hz). J = 5.8, 14.5 Hz), 3.84 (s, 3H), 4.22-4.25 (m, 1H), 6.58 (dd, 1H, J = 2.2, 8.0 Hz), 6.69 (d, 1H, J = 2.1 Hz), 6.77 (d, 1H, J = 8.0 Hz). esims (m/z): 212.7 ([M + H]+). 423.2 ([2M + H]+), 210.2 ([M - H]-), 241.1 ([M + Cl]-).

59-92-7
709 suppliers
$14.00/5g

1421-65-4
127 suppliers
$5.00/250mg
Yield:-
Reaction Conditions:
with thionyl chloride in methanol;toluene
Steps:
131 3-(3,4-Dihydroxyphenyl)-L-alanine methyl ester hydrochloride
3-(3,4-Dihydroxyphenyl)-L-alanine methyl ester hydrochloride A solution of 1.97 g (10 mmol) of L-DOPA 15 in 100 ml of MeOH at 0° C. was added 6.57 ml (90 mmol) of thionyl chloride via addition funnel. The mixture was stirred overnight while the temperature was slowly warmed to room temperature. The solvent was evaporated and the thick oil was treated with toluene (3*15 ml) and evaporated. The yield of white solid was 3.26 g (100%); NMR (DMSQ-d6) δ 2.90 (m, 2H), 3.38 (bs, 2H), 3.61 (s, 3H), 4.08 (m, 1H), 6.40 (d, 1H, J=7 Hz), 6.59 (s, 1H), 6.65(d, 2H, J=7 Hz), 8.59 (bs, 1H), 8.90 (d, 1H, J=10 Hz). MS: 212 m/z (M+H)+.
References:
Cephalon, Inc. US5952328, 1999, A

67-56-1
782 suppliers
$7.29/5ml-f

59-92-7
709 suppliers
$14.00/5g

1421-65-4
127 suppliers
$5.00/250mg

59-92-7
709 suppliers
$14.00/5g
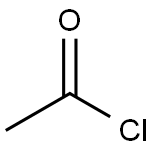
75-36-5
592 suppliers
$17.92/100G

1421-65-4
127 suppliers
$5.00/250mg
