
5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione synthesis
- Product Name:5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione
- CAS Number:150728-12-4
- Molecular formula:C15H12N4O4
- Molecular Weight:312.28

20730-58-9

16879-48-4
![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione](/CAS/GIF/150728-12-4.gif)
150728-12-4
Step 3: Synthesis of 5-(2-methoxyphenoxy)-2-(pyrimidin-2-yl)pyrimidine-4,6(1H,5H)-dione Sodium (0.69 g, 30 mmol) was dissolved in anhydrous ethanol (40 mL) under nitrogen protection, followed by the addition of diethyl 2-(2-methoxyphenoxy)malonate (2.82 g, 10 mmol) and 2-amidinopyrimidinium benzenesulfonate (2.8 g, 10 mmol) to an ethanol (30 mL) solution, in that order. The reaction mixture was heated to reflux for 16 hours. Upon completion of the reaction, the mixture was poured into ice water, acidified with 3M hydrochloric acid to pH ≈ 2, and then concentrated until a precipitate precipitated. The precipitate was collected by filtration, washed with water and dried to give 804 mg of yellow solid product in 25% yield. Product characterization data. 1H NMR (300 MHz, DMSO-d6) δ 3.84 (s, 3H), 6.68 (d, J = 6.9 Hz, 1H), 6.77 (t, J = 7.5 Hz, 1H), 6.91 (t, J = 7.5 Hz, 1H), 7.05 (d, J = 6.9 Hz, 1H), 9.05 (d, J = 5.1 Hz, 2H), 12.19 (br, 2H). 12.19 (br, 2H). ESI-MS, m/z = 313 (M + H+). Note: 5-(2-methoxyphenoxy)-[2,2'-dipyrimidinyl]-4,6(1H,5H)-dione can be prepared with reference to the method described in Example 1 step 3.
Yield: 25%
Reaction Conditions:
with ethanol;sodium for 16 h;Heating / reflux;
Steps:
1.3; 2.3
Step 3; 5-(2-methoxyphenoxy)-2-(pyrimidin-2-yl)pyrimidine-4,6(1H,5H)-dione: 2-(2-methoxyphenoxy) propanedioic acid diethyl ester (2.82 g, 10 mmol) and a solution of 2-amidinopyrimidine benzenesulfonate (2.8 g, 10 mmol) in ethanol (30 mL) were sequentially added to sodium (0.69 g, 30 mmol) dissolved in absolute ethanol (40 mL). The reaction was heated at reflux for about 16 hours, poured onto ice-water, acidified with 3M hydrochloric acid, and concentrated until a precipitate formed. The precipitate was collected by filtration, washed with water and dried to yield 804 mg of desired product as a yellow solid. (25% yield). 1H NMR (300 MHz, DMSO-d6) δ 3.84 (s, 3H), δ 6.68 (d, J=6.9 Hz, 1H), δ 6.77 (t, J=7.5 Hz, 1H), δ 6.91 (t, J=7.5 Hz, 1H), 7.05 (d, J=6.9 Hz, 1H), 9.05 (d, J=5.1 Hz, 2H), 12.19 (br, 2H); ESI-MS, m/z=313 (M+H+).Step 3 5-(2-methoxyphenoxy)-2-(pyrimidin-2-yl)pyrimidine-4,6(1H,5H)-dione: The title compound was made by following the procedure set forth in Example 1, step 3.
References:
AUSPEX PHARMACEUTICALS, INC. US2008/242687, 2008, A1 Location in patent:Page/Page column 32; 34
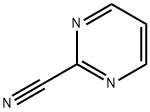
14080-23-0
398 suppliers
$7.00/500mg

150726-89-9
139 suppliers
$6.00/5g
![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione](/CAS/GIF/150728-12-4.gif)
150728-12-4
153 suppliers
$11.00/1g

90-05-1
787 suppliers
$5.00/1g
![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione](/CAS/GIF/150728-12-4.gif)
150728-12-4
153 suppliers
$11.00/1g

57871-18-8
2 suppliers
inquiry
![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione](/CAS/GIF/150728-12-4.gif)
150728-12-4
153 suppliers
$11.00/1g

14080-23-0
398 suppliers
$7.00/500mg
![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6(1H,5H)-dione](/CAS/GIF/150728-12-4.gif)
150728-12-4
153 suppliers
$11.00/1g