
(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate synthesis
- Product Name:(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate
- CAS Number:174649-09-3
- Molecular formula:C15H19FN2O6S
- Molecular Weight:374.38

124-63-0

168828-82-8
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
To a 1L solution of dichloromethane containing 132.8 g of (R)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-hydroxymethyl-2-oxazolidinone and 87.1 g of triethylamine was slowly added 74 g of methanesulfonyl chloride at 0°C under nitrogen protection. The reaction mixture was stirred at 0 °C for 30 min and then gradually warmed up to room temperature. A white solid precipitated during the reaction. Subsequently, a solvent mixture consisting of 1.75 L of water, 6.50 L of dichloromethane and 1 L of ethyl acetate was added to the reaction system and stirring was continued for 30 minutes. Upon completion of the reaction, the white solid product was collected by filtration. A final product of 140 g was obtained with a yield of 105.42% (w/w).

124-63-0
0 suppliers
$10.00/5g

168828-82-8
248 suppliers
$5.00/250mg
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 0.5 h;
Steps:
5
To a solution of 132.8 g of (R)-N-[[3-[3-fluoro-4-morpholinyl]phenyl]-2-oxo-5- oxazolidinyl] methanol and 87.1 g of triethylamine in IL of methylene dichloride at O0C under nitrogen was added 74 gm of methanesulfonyl chloride. The reaction mixture was allowed to stir at 0°C for 30 min, then allowed to warm to ambient temperature. A white solid was precipitated. 1.75 L water, 6.50 L methylene dichloride and IL ethyl acetate mixture were added followed by stirring for 30 min. The reaction mass was filtered to get a white solid. Yield: 140 g.; Percentage: 105.42% w/w
References:
USV LIMITED WO2009/63505, 2009, A2 Location in patent:Page/Page column 14

369-34-6
419 suppliers
$5.00/5g
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg

2689-39-6
159 suppliers
$10.00/1g
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg
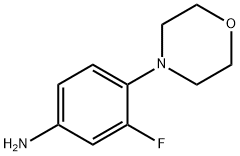
93246-53-8
353 suppliers
$8.00/5g
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg
-, phenylmethyl ester (9CI)](/CAS/20210111/GIF/912552-55-7.gif)
912552-55-7
1 suppliers
inquiry
![(R)-[3-(3-Fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl methanesulfonate](/CAS2/GIF/174649-09-3.gif)
174649-09-3
157 suppliers
$39.00/25mg