
(4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid synthesis
- Product Name:(4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
- CAS Number:196404-55-4
- Molecular formula:C22H25NO6
- Molecular Weight:399.44

1531632-32-2

196404-55-4
The (4S,5R)-3-(tert-butoxycarbonyl)-2-(4-methoxyphenyl)-4-phenyloxazolidine-5-carboxylic acid precursor obtained in the previous step (4.83 g, 9.88 mmol) was dissolved in 10 mL of methanol and 1.0 g of palladium hydroxide was added as a catalyst. Hydrogen was introduced into the reaction system at room temperature (pressure maintained at 20 psi) and the reaction was carried out for 1 h, during which the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was filtered to remove the catalyst, and the filtrate was concentrated under reduced pressure and purified by column chromatography (eluent ratio of petroleum ether/ethyl acetate = 5:1) to afford (4S,5R)-3-(tert-butoxycarbonyl)-2-(4-methoxyphenyl)-4-phenyl-oxazolidine-5-carboxylic acid as a white solid (2.68 g, 67.9% yield).

670254-71-4
39 suppliers
inquiry

196404-55-4
197 suppliers
$8.00/250mg
Yield:196404-55-4 92%
Reaction Conditions:
with methanol;sodium hydroxide at 20;
Steps:
6 Synthesis of benzooxazoline carboxylic acid
The above mention product 7 dissolved with ΜeOΗ, added dilute NaOH and stir at room temperature, TLC has been monitored after completion of the reaction and remove MeOH, the residue is diluted with Ac0Et / H20, static stratification. Into the aqueous phase added the AcOEt, and adjust the pH with dilute hydrochloric acid. The mixture is allowed to stand for separation, the organic phase has been separated, and the aqueous phase has been extracted with AcOEt, the combined organic phases are washed with water, dried over anhydrous MgS04, the solvent has been removed, and then obtained benzooxazoline carboxylic acid 8, yield of both steps is 92%.
References:
Jilin Pharmaceutical College;Fourth Military Medical University;Zhang Xiurong;Zhang Shengyong;Chen Ming;Jin Ying;Liang Chengwu;Chang Sheng;Li Xiaoye;Liu Xueying;Chen Weiping;Guan Shaoyu CN106632297, 2017, A Location in patent:Paragraph 0021

1531632-32-2
1 suppliers
inquiry

196404-55-4
197 suppliers
$8.00/250mg
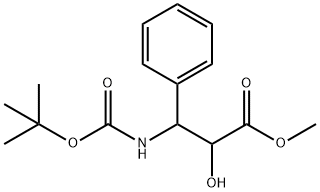
124605-42-1
175 suppliers
$8.00/250mg

196404-55-4
197 suppliers
$8.00/250mg

157240-36-3
18 suppliers
inquiry

196404-55-4
197 suppliers
$8.00/250mg
![2-Propanesulfinamide, 2-methyl-N-(phenylmethylene)-, [S(R)]-](/CAS/20210305/GIF/196929-85-8.gif)
196929-85-8
0 suppliers
inquiry

196404-55-4
197 suppliers
$8.00/250mg