
(1R,2S,5R)-(-)-MENTHYL (S)-P-TOLUENESULFINATE synthesis
- Product Name:(1R,2S,5R)-(-)-MENTHYL (S)-P-TOLUENESULFINATE
- CAS Number:1517-82-4
- Molecular formula:C17H26O2S
- Molecular Weight:294.45
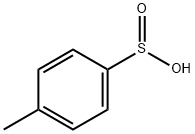
536-57-2

2216-51-5

1517-82-4
The general procedure for the synthesis of (S)-((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 4-methylbenzenesulfinyl ester from p-toluenesulfinic acid and L-menthol was as follows: to a 250 mL flame-dried and N2 purged round-bottomed flask were added sequentially p-toluenesulfinic acid (0.525 g, 3.36 mmol), 4-bromobenzyl alcohol (0.598 g, 3.20 mmol) and DMAP (0.078 g, 0.64 mmol), which were then dissolved in anhydrous CH2Cl2 (14 mL). To this solution was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.644 g, 3.36 mmol) in a single addition and the reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the reaction mixture was diluted with CH2Cl2 (30 mL) and washed sequentially with 1M HCl (30 mL) and brine (30 mL). The organic layer was separated, dried with MgSO4, filtered and the solvent was removed under reduced pressure. Finally, the crude product was purified by fast column chromatography.

536-57-2
152 suppliers
$34.00/1g

2216-51-5
704 suppliers
$5.00/100mg

1517-82-4
123 suppliers
$21.00/1g
Yield: 99%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 25; for 16 h;Inert atmosphere;Temperature;Reagent/catalyst;
Steps:
4-Bromobenzyl p-Toluenesulfinate (1c), Typical Procedure
General procedure: To a 250 mL flame-dried N2 purged round-bottomed flask was added p-toluenesulfinic acid (0.525 g, 3.36 mmol), 4-bromobenzyl alcohol (0.598 g, 3.20 mmol), and DMAP (0.078 g, 0.64 mmol) sequentially and dissolved in anhyd CH2Cl2 (14 mL). To the solution was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.644 g, 3.36 mmol) in one portion and stirred for 16 h at r.t. The reaction mixture was diluted with CH2Cl2 (30 mL) and washed with aq 1 M HCl (30 mL) and brine (30 mL). The organic layer was dried (MgSO4), filtered, and solvents removed under reduced pressure. The crude product was purified by flash column chromatography .
References:
Gafur, Sayed Habibul;Waggoner, Stephanie L.;Jacobsen, Eric;Hamaker, Christopher G.;Hitchcock, Shawn R. [Synthesis,2018,vol. 50,# 24,art. no. SS-2018-M0396-OP,p. 4855 - 4866]

10439-23-3
22 suppliers
$56.54/50mgs:

2216-51-5
704 suppliers
$5.00/100mg

98147-48-9
0 suppliers
inquiry

1517-82-4
123 suppliers
$21.00/1g

2216-51-5
704 suppliers
$5.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1517-82-4
123 suppliers
$21.00/1g

184636-51-9
2 suppliers
inquiry

2216-51-5
704 suppliers
$5.00/100mg
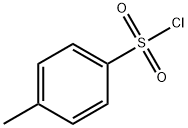
98-59-9
629 suppliers
$9.00/5g

1517-82-4
123 suppliers
$21.00/1g

2216-51-5
704 suppliers
$5.00/100mg

19158-51-1
177 suppliers
$52.00/1 g

1517-82-4
123 suppliers
$21.00/1g