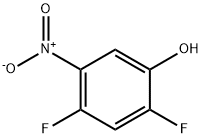
2,4-Difluoro-5-nitrophenol synthesis
- Product Name:2,4-Difluoro-5-nitrophenol
- CAS Number:113512-57-5
- Molecular formula:C6H3F2NO3
- Molecular Weight:175.09

152169-43-2

113512-57-5
Using 2,4-difluorophenyl ethyl carbonate (3.1 g, 16 mmol) as raw material, nitric acid (0.78 ml, 19 mmol) was slowly added to a solution of sulfuric acid (10 mL) at 0 °C, keeping the reaction temperature at about 0 °C. After 15 min of reaction, the reaction was terminated by adding ice-cold water (70 mL), and the product was extracted with ethyl acetate (2 × 50 mL). The organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and concentrated to give the nitro product as a thick syrup. The nitro product was dissolved in methanol (20 mL), solid sodium bicarbonate (4.0 g, 47 mmol) was added and stirred for 16 hours at room temperature. After completion of the reaction, the mixture was filtered and the filtrate was concentrated. The resulting solid was dissolved in water (20 ml) and acidified with 3M HCl solution to pH~5. The product was extracted with dichloromethane (3 x 25 mL), the organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, and concentrated to give 2,4-difluoro-5-nitrophenol (2.34 g, 84% yield). The product was confirmed by 1H NMR (400 MHz, acetone-d6): δ 9.59 (s, 1H), 7.78 (t, J=7.2 Hz, 1H), 7.45 (t, J=10.4 Hz, 1H); mass spectrometry (ESI) m/z: 176.0 (M+H+).

152169-43-2
5 suppliers
$75.00/100mg

113512-57-5
85 suppliers
$15.00/1g
Yield: 84%
Reaction Conditions:
Stage #1:2,4-difluorophenyl ethyl carbonate with sulfuric acid;nitric acid at 0; for 0.25 h;
Stage #2: with sodium hydrogencarbonate in methanol at 20; for 16 h;
Steps:
A5
To a solution of 2,4-difluorophenyl ethyl carbonate (3.1 g, 16 mmol) in sulphuric acid (10 mL) was added fuming HN03 (0.78 ml, 19 mmol) slowly, keeping the internal temperature around 0 °C. After 15 min ice cold water (70 mL) was added, the product was extracted with ethyl acetate (2x50 mL), the combined organic layers were washed with brine, dried (Na2SC>4) and concentrated to afford the nitro product as a thick syrup. This nitro product was dissolved in methanol (20 mL) and to this solution was added solid NaHC03 (4.0 g, 47 mmol) and the resultant mixture was stirred for 16h at RT. The mixture was filtered and the filtrate was concentrated. The resulting solid was dissolved in water (20 ml) and acidified with 3M HC1 solution to pH~5. The product was extracted with CH2CI2 (3x25 mL), the combined organic layers were washed with brine, dried ( a2S04) and concentrated to afford 2,4-difluoro-5-nitrophenol (2.34 g, 84% yield). 1H NMR (400 MHz, Acetone-i 6) δ 9.59 (s, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.45 (t, J = 10.4 Hz, 1H); MS (ESI) m/z: 176.0 (M+H+).
References:
DECIPHERA PHARMACEUTICALS, LLC;FLYNN, Daniel L.;PETILLO, Peter A.;KAUFMAN, Michael D. WO2013/36232, 2013, A2 Location in patent:Paragraph 00224

367-27-1
349 suppliers
$6.00/5g

113512-57-5
85 suppliers
$15.00/1g