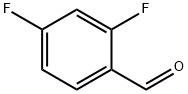
2,4-Difluorobenzaldehyde synthesis
- Product Name:2,4-Difluorobenzaldehyde
- CAS Number:1550-35-2
- Molecular formula:C7H4F2O
- Molecular Weight:142.1

452-76-6

1550-35-2
(1) Device: Refer to Figure 2 to configure the tubular reactor system. The reactor adopts (3a + 3b) type DC channel and round cake pulse diameter adjustable rectangular flat tube combination design, the specific tube diameter and volume need to be calculated according to the reactant flow and the required residence time to determine, the heat transfer medium selection of heat transfer oil. (2) Preparation of catalyst solution: dissolve 6.06 g of cobalt acetate and 6.06 g of sodium molybdate in 200 mL of 2,4-difluorotoluene and 200 mL of acetic acid, respectively, and mix them well to ensure that n (cobalt acetate): n (2,4-difluorotoluene) = 0.015: 1. Preparation of oxidizer solution: dissolve 6.06 g of sodium bromide in 30% H2O2 to form a solution of H2O2-acetic acid. . Subsequently, 2,4-difluorotoluene-acetic acid solution and H2O2-acetic acid solution were injected into a tubular reactor via a constant flow pump at a flow rate of 5.33 mL/min and 10.67 mL/min, respectively, controlling n(H2O2): n(2,4-difluorotoluene) = 2: 1. The reaction was carried out in a microchannel reactor (shown in Fig. 2) at a temperature maintained at 105 °C and a residence time of 400 s. After completion of the reaction, the outlet material was cooled to 0 °C and quenched with dichloromethane. The conversion of 2,4-difluorotoluene was 49.5% and the yield of 2,4-difluorobenzaldehyde was 31.1% by GC analysis.
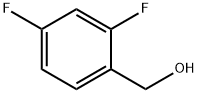
56456-47-4
202 suppliers
$5.00/1g

1550-35-2
392 suppliers
$6.00/5g
Yield:1550-35-2 89%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;copper diacetate in water;acetonitrile at 20; for 6 h;Green chemistry;
Steps:
General procedure: A mixture of alcohol (5.0 mmol), Cu(OAc)2 (9.1 mg, 0.05 mmol), and TEMPO (7.8 mg, 0.05 mmol) in CH3CN/H2O (5/10 mL) was stirred at room temperature for specified time. After completion of the reaction (monitored by TLC, eluents: petroleum ether/ethyl acetate = 4/1), dichloromethane (10 mL) was added to the resulting mixture. The dichloromethane phase was separated, and the aqueous phase was further extracted with dichloromethane (10 mL × 2). The combined organic layers were dried over anhydrous sodium sulfate and concentrated to give a residue, which was purified by column chromatography (eluents: petroleum ether/ethyl acetate = 10/1) to provide the desired product.
References:
Jiang, Jian-An;Du, Jia-Lei;Wang, Zhan-Guo;Zhang, Zhong-Nan;Xu, Xi;Zheng, Gan-Lin;Ji, Ya-Fei [Tetrahedron Letters,2014,vol. 55,# 10,p. 1677 - 1681] Location in patent:supporting information

452-76-6
249 suppliers
$8.00/5g

1550-35-2
392 suppliers
$6.00/5g

64-18-6
1127 suppliers
$22.12/250ML

372-18-9
429 suppliers
$9.00/1g

1550-35-2
392 suppliers
$6.00/5g

348-57-2
430 suppliers
$5.00/5g

1550-35-2
392 suppliers
$6.00/5g
