
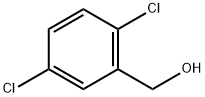
2,5-Dichlorobenzaldehyde synthesis
- Product Name:2,5-Dichlorobenzaldehyde
- CAS Number:6361-23-5
- Molecular formula:C7H4Cl2O
- Molecular Weight:175.01
34145-05-6

6361-23-5
General procedure for the synthesis of 2,5-dichlorobenzaldehyde from 2,5-dichlorobenzenemethanol: 2,5-dichlorobenzenemethanol (1 mmol), water (1 mL), and CoFe2O4 magnetic nanoparticles (11.8 mg, 5 mol%) were added to a round bottom flask. The reaction mixture was stirred for 2 min and then Oxone (0.6 mmol) was added in three batches. The reaction mixture was stirred at room temperature for the indicated time (refer to Table 5). The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent ethyl acetate-cyclohexane, 2:10, v/v). After completion of the reaction, the product was extracted with dichloromethane. The solvent was removed by distillation under reduced pressure to obtain the crude product. The crude product was purified using silica gel column chromatography to obtain pure 2,5-dichlorobenzaldehyde. Finally, the aliphatic by-products in dichloromethane were dried with anhydrous magnesium sulfate and analyzed by gas chromatography-flame ionization detector (GC-FID).

34145-05-6
119 suppliers
$6.00/250mg

6361-23-5
214 suppliers
$8.00/5g
Yield:6361-23-5 88%
Reaction Conditions:
Stage #1:2,5-dichlorobenzyl alcohol with cobalt ferrite in water for 0.0333333 h;
Stage #2: with oxone(R) in water at 20; for 0.75 h;
Steps:
General Procedure for the Oxidation of Alcohol
General procedure: Alcohol (1 mmol), water (1 mL), and CoFe2O4 MNPs (11.8mg, 5 mol %) were added to a round-bottomed flask. The reaction mixture was stirred for the two minutes, and then oxone (0.6 mmol) was added in three portions. The reaction mixture was placed at room temperature and stirred for the specified time (Table 5). The reaction was followed by TLC (EtOAc-cyclohexane, 2:10). After the completion of the reaction, the product was extracted in dichloromethane. The solvent was evaporated under reduced pressure to give the corresponding aromatic products. Purification of the residue using plate chromatography (silica gel) provided the pure carbonyl compounds. The aliphatic products in dichloromethane was dried with anhydrous MgSO4 and detected by GC-FID.
References:
Sadri, Fariba;Ramazani, Ali;Massoudi, Abdolhossain;Khoobi, Mehdi;Azizkhani, Vahid;Tarasi, Roghayeh;Dolatyari, Leila;Min, Bong-Ki [Bulletin of the Korean Chemical Society,2014,vol. 35,# 7,p. 2029 - 2032]

587-04-2
428 suppliers
$7.00/25g

6361-23-5
214 suppliers
$8.00/5g

19398-61-9
132 suppliers
$10.00/5 g

6361-23-5
214 suppliers
$8.00/5g

2745-49-5
112 suppliers
$75.00/1g

6361-23-5
214 suppliers
$8.00/5g

3084-17-1
0 suppliers
inquiry

6361-23-5
214 suppliers
$8.00/5g