
2,6-di-tert-butylpyrimidin-4(3H)-one synthesis
- Product Name:2,6-di-tert-butylpyrimidin-4(3H)-one
- CAS Number:69050-79-9
- Molecular formula:C12H20N2O
- Molecular Weight:208.3
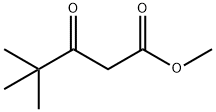
55107-14-7
206 suppliers
$10.00/1g

69050-79-9
8 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: pivalamidine hydrochloridewith sodium methylate in methanol;ethanol at 20; for 0.5 h;
Stage #2: Methyl 4,4-dimethyl-3-oxopentanoate in methanol;ethanol at 78; for 3 h;
Steps:
25.1
2,2-Dimethylpropanimidamide hydrochloride (1.0 g, 7.3 mmol) was dissolved in ethyl alcohol (18 mL), sodium methoxide (0.5 M in methanol, 29 mL) added and the solution stirred at RT for 30 minutes. Methyl 4,4-dimethyI-3-oxovalerate (1.2 g, 7.3 mmol) was added and the solution stirred at 78 0C for 3 hours. The mixture was cooled to RT, poured into water (500 mL), acidified with acetic acid, followed by aqueous/EtOAc work-up to afford the titled compound.
References:
WO2007/64553,2007,A2 Location in patent:Page/Page column 70