
2-Acetyl-5-bromothiophene synthesis
- Product Name:2-Acetyl-5-bromothiophene
- CAS Number:5370-25-2
- Molecular formula:C6H5BrOS
- Molecular Weight:205.07

88-15-3

5370-25-2
The general procedure for the synthesis of 2-acetyl-5-bromothiophene from 2-acetylthiophene was as follows: 2-acetylthiophene (1.08 mL, 10 mmol), N-bromosuccinimide (NBS) (1.78 g, 30 mmol), and acetic anhydride (3.78 mL, 40 mmol) were added sequentially to a dry, 25 mL round-bottomed flask, which was equipped with a protective tube. Acetic acid (0.40 mL) was subsequently added to the mixture. The reaction mixture was stirred at 50 °C for 1 h protected from light and the color of the solution was observed to change from colorless to light yellow. Upon completion of the reaction, the mixture was cooled to room temperature and poured into 100 mL of water with continuous stirring until the acetic anhydride was completely hydrolyzed. At this point, 2-acetyl-5-bromothiophene precipitated as white crystals. The product was collected by filtration and washed in batches with 50 mL of water to give a final yield of 82%. The melting point of the product was 92-94 °C (acetone).1H NMR (300 MHz, CDCl3) data were as follows: δ 7.40 (d, J=3.9 Hz, 1H), 7.08 (d, J=3.9 Hz, 1H), 2.48 (s, 3H).

1003-09-4
488 suppliers
$10.00/25g
75-36-5
592 suppliers
$17.92/100G

5370-25-2
273 suppliers
$8.00/5g
Yield:5370-25-2 86.1%
Reaction Conditions:
in dichloromethane
Steps:
R.1 2-Acetyl-5-bromothiophene (b-1) STR41
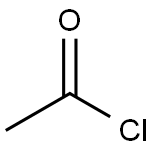
REFERENTIAL EXAMPLE 1 2-Acetyl-5-bromothiophene (b-1) STR41 When 1.56 ml (1.1 equivalents) of acetyl chloride was added to a solution of 3.26 g (0.02 mmol) of 2-bromothiophene in 30 ml of CH2 Cl2, the mixture foamed and turned black. After stirring for 1 hr. at room temperature, the mixture was mixed with ice and concentrated hydrochloric acid. While being decolored with active carbon, the mixture was extracted with CH2 Cl2, whereby 3.53 g (Yield: 86.1%) of the objective product, 2-acetyl-5-bromothiophene (b-1) was obtained as colorless crystals.
References:
Shinogi & Co., Ltd. US4839365, 1989, A

88-15-3
614 suppliers
$5.00/10g

5370-25-2
273 suppliers
$8.00/5g

1003-09-4
488 suppliers
$10.00/25g

108-24-7
0 suppliers
$14.00/250ML

5370-25-2
273 suppliers
$8.00/5g

1003-09-4
488 suppliers
$10.00/25g

64-19-7
1602 suppliers
$10.00/25ML

5370-25-2
273 suppliers
$8.00/5g
