
2-Amino-3-benzyloxypyridine synthesis
- Product Name:2-Amino-3-benzyloxypyridine
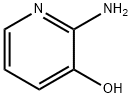
- CAS Number:24016-03-3
- Molecular formula:C12H12N2O
- Molecular Weight:200.24
16867-03-1

100-44-7

24016-03-3
At a temperature lower than 40°C, 48L of deionized water was added to a 300L stainless steel reactor, and 48kg of sodium hydroxide was added under slow stirring until completely dissolved. Subsequently, 16 kg of 2-amino-3-hydroxypyridine, 1.6 kg of tetrabutylammonium bromide, and 17.92 L of benzyl chloride were added sequentially to the sodium hydroxide solution. The reaction mixture was heated to 70~75°C and kept at this temperature for 6 hours. When the reaction was completed, stirring was stopped and the mixture was left to stratify. The aqueous phase was extracted with toluene (20L x 3) and the combined organic phases were washed with pure water (30L x 2). The organic phase was concentrated until a large amount of solid precipitated, followed by crystallization at 0~5°C and stirring for 2 hours. Upon completion of crystallization, centrifugation was performed and the filter cake was washed with 3.2 L of pre-cooled toluene. Finally, it was dried under vacuum at 50~55°C for 3 h to give 22.5 kg of bright yellow solid Intermediate II (2-amino-3-benzyloxypyridine) in 77.3% molar yield and 99.53% purity.

16867-03-1
585 suppliers
$10.00/25g

100-44-7
658 suppliers
$13.50/250G

24016-03-3
360 suppliers
$5.00/1g
Yield:24016-03-3 77.3%
Reaction Conditions:
with tetrabutylammomium bromide;sodium hydroxide in water at 40 - 75; for 6 h;Large scale;
Steps:
1 Example 1: Intermediate II: Preparation of 2-amino-3-benzyloxypyridine
temperature below 40 ° C, to the 300L stainless steel reactor 48L of purified water was added slowly stirring 48kg sodium hydroxide, stirring until dissolved.To sodium hydroxide solution followed by adding 16kg 2-amino-3-hydroxypyridine, 1.6kg tetrabutylammonium bromide, 17.92L benzyl chloride, plus warming to 70 ~ 75 ° C reaction 6 hours, stirring was stopped,Let stand, liquid separation; aqueous phase extraction with toluene (20Lx3);Purified water washing (30Lx2), the organic phase was collected, concentrated to a large amount of solid precipitation, temperature 0 ~ 5 ° C, crystallization was stirred for 2 hours, centrifuged,The filter cake was rinsed with 3.2 L of pre-cooled toluene and dried under vacuum at 50~55 ° C. for 3 hours to afford Intermediate II as a bright yellow solid, 22.5 kg of 2-amino-3-benzyloxypyridine, with a molar yield of 77.3% , Purity 99.53%.
References:
Jinan Cornwall, Ontario Pharmaceutical Technology Co., Ltd.;Zhang Dongmei;Zhang Ying;Xu Jiaping;Ai Leifeng;Zheng Liangwen;Sun Limeng CN107311998, 2017, A Location in patent:Paragraph 0035-0036

16867-03-1
585 suppliers
$10.00/25g

100-39-0
434 suppliers
$10.00/10 g

24016-03-3
360 suppliers
$5.00/1g

16867-03-1
585 suppliers
$10.00/25g

24016-03-3
360 suppliers
$5.00/1g