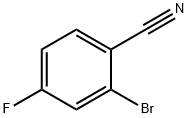
2-Bromo-4-fluorobenzonitrile synthesis
- Product Name:2-Bromo-4-fluorobenzonitrile
- CAS Number:36282-26-5
- Molecular formula:C7H3BrFN
- Molecular Weight:200.01
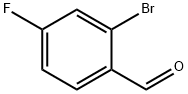
59142-68-6

36282-26-5
General procedure for the synthesis of 2-bromo-4-fluorobenzonitrile from 2-bromo-4-fluorobenzaldehyde: In a dry 2 mL reaction vial, 2-bromo-4-fluorobenzaldehyde (3a, 0.136 g, 1 mmol, 1 eq.) and pyridine (0.474 g, 6.0 mmol, 6 eq.) were added followed by the addition of acetonitrile (2 mL) to make the concentration of 3a to be 0.5 M. Next, to the reaction vial was added Ru(bpy)3(PF6)2 (0.017 g, 0.02 mmol, 0.02 eq.), 2 (0.043 g, 0.20 mmol, 0.20 eq.), (NH4)2S2O8 (0.501 g, 2.2 mmol, 2.2 eq.), and activated 3?molecular sieves (~0.2 g). The reaction vial was sealed and placed in a blue LED reactor for 24 hours of irradiation. The temperature of the mixture was maintained at ~50 °C during the reaction without fan cooling. Upon completion of irradiation, the reaction was quenched with EtOAc and the mixture was transferred to a dispensing funnel. Additional EtOAc (30 mL) and 0.5 M HCl (aq, 30 mL) were added to separate the organic and aqueous layers. The aqueous layer was extracted with EtOAc (3 × 20 mL), and the combined organic layers were washed sequentially with 0.5 M HCl (aq, 2 × 20 mL), saturated aqueous NaHCO3 (2 × 20 mL), and brine (20 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain the crude product. The crude product was adsorbed on silica gel (the amount of silica gel was 1.5 times the weight of the theoretical product) and gently loaded onto a silica gel column. The target product was first rinsed with excess hexane (about 5 times the volume of the column), and then eluted with a hexane/EtOAc solvent mixture (3-4 times the volume of the column) with a volume ratio of 90:10. The eluate was concentrated by rotary evaporation to give pure 2-bromo-4-fluorobenzonitrile (3c, 0.066 g, 50% yield) as a white solid.

1006-41-3
323 suppliers
$6.00/5g

36282-26-5
208 suppliers
$7.00/5g
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: thionyl chloride / 3 h / Reflux; Inert atmosphere
1.2: 16 h / 20 °C
2.1: thionyl chloride / 3 h / Reflux; Inert atmosphere
References:
Donaldson, Lauren R.;Wallace, Stephen;Haigh, David;Patton, E. Elizabeth;Hulme, Alison N. [Organic and Biomolecular Chemistry,2011,vol. 9,# 7,p. 2233 - 2239]

59142-68-6
277 suppliers
$14.00/25g

36282-26-5
208 suppliers
$7.00/5g

1006-40-2
37 suppliers
$26.30/250mg

36282-26-5
208 suppliers
$7.00/5g

1309606-40-3
1 suppliers
inquiry

36282-26-5
208 suppliers
$7.00/5g
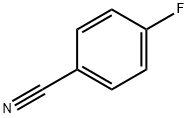
1194-02-1
521 suppliers
$6.00/10g

36282-26-5
208 suppliers
$7.00/5g