
2-Bromo-5-iodopyridine synthesis
- Product Name:2-Bromo-5-iodopyridine
- CAS Number:73290-22-9
- Molecular formula:C5H3BrIN
- Molecular Weight:283.89

20511-12-0

73290-22-9
General procedure for the synthesis of 2-bromo-5-iodopyridine from 2-amino-5-iodopyridine: Bromine (3 mL) was slowly added dropwise to a mixture of 2-amino-5-iodopyridine (5 g, 20 mmol) dissolved in 48% aqueous hydrobromic acid (10 mL), and the reaction system was cooled by an ice bath to maintain the reaction at low temperature. Subsequently, sodium nitrite (3.4 g, dissolved in 5 mL of water) solution was added dropwise, and the reaction temperature was controlled not to exceed 15 °C. After the dropwise addition, a solution of sodium hydroxide (16 g) in water (40 mL) was added. A brown solid precipitated from the reaction mixture and was extracted with dichloromethane (50 mL). The organic phases were combined, washed sequentially with water and saturated saline, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to afford the target product 2-bromo-5-iodopyridine (4.4 g, 78% yield).
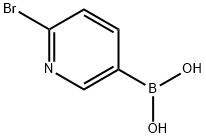
223463-14-7
247 suppliers
$6.00/250mg

73290-22-9
339 suppliers
$6.00/1g
Yield:73290-22-9 79%
Reaction Conditions:
with perfluoroisopropyl iodide;copper;hydroquinone in N,N-dimethyl-formamide at 20; for 24 h;
Steps:
1 4.1. Typical procedure for the iodination of 1a-t by (CF3)2CFI
General procedure: (4-Nitrophenyl)boronic acid (0.067 g, 0.4 mmol), copper powder (0.0052 g, 0.08 mmol,), (CF3)2CFI (0.178 g, 0.6 mmol), and DMF (2 mL) were placed in a closed tube with a rubber stopper. The mixture was reacted at room temperature equipped with an air balloon for 24 h. The resulting suspension was poured into water and extracted with ethyl acetate. The organic layer was dried over anhydrous Na2SO4 and concentrated to dryness. The crude product was purified by flash column chromatography on silica gel using petroleum ether/ethyl acetate = 20: 1 (v/v) as eluent to give 0.086 g of 2j as a light yellow solid (0.35 mmol, 87%).
References:
Liu, Xi-Hai;Leng, Jing;Jia, Su-Jiao;Hao, Jian-Hong;Zhang, Fanglin;Qin, Hua-Li;Zhang, Cheng-Pan [Journal of Fluorine Chemistry,2016,vol. 189,p. 59 - 67]

20511-12-0
462 suppliers
$6.00/1g

73290-22-9
339 suppliers
$6.00/1g

624-28-2
718 suppliers
$5.00/5g

73290-22-9
339 suppliers
$6.00/1g

13534-97-9
437 suppliers
$6.00/1g

73290-22-9
339 suppliers
$6.00/1g

624-28-2
718 suppliers
$5.00/5g

73290-22-9
339 suppliers
$6.00/1g

223463-13-6
383 suppliers
$5.00/1g