
2-CHLORO-7-NITROQUINOXALINE synthesis
- Product Name:2-CHLORO-7-NITROQUINOXALINE
- CAS Number:55686-94-7
- Molecular formula:C8H4ClN3O2
- Molecular Weight:209.59

89898-96-4

55686-94-7
The general procedure for the synthesis of 2-chloro-7-nitroquinoxaline from 7-nitro-2(1H)-quinoxalinone was as follows: in a round-bottomed flask, 7-nitroquinoxalin-2-ol (12.73 g, 67 mmol) was dissolved in 20 mL of phosphorochloridic acid (POCl3), and the mixture was heated and refluxed for 3 hours. Upon completion of the reaction, the reaction mixture was quenched by slowly pouring it into a stirred ice-water mixture. Subsequently, the precipitate was collected by filtration and washed with cold water to give 13.43 g of red to cherry colored solid product in 96% yield. The melting point of the product was 183-186 °C (literature value: 185-189 °C). Analyzed by ATR/FTIR spectroscopy, the main absorption peaks were located at 3092, 3048, 1682, 1525, 1350 and 740 cm?1. 1H NMR (200 MHz, DMSO-d6) δ: 9.22 (s, 1H, H3), 8.88 (d, 1H, J = 3.0 Hz, H8), 8.59 (dd, 1H, J = 2.0 , 9.0 Hz, H6), 8.39 (d, 1H, J = 9.0 Hz, H5).13C NMR (100 MHz, DMSO-d6) δ: 149.2, 148.8, 148.3, 142.8, 140.2, 130.9, 124.1, 123.9.DEPT-135 (100 MHz, DMSO-d6) δ : 148.8, 130.9, 124.1, 123.9. mass spectrometry analysis showed molecular ion peaks of 209.1 and 211.1.

89898-96-4
61 suppliers
$286.00/5g

55686-94-7
64 suppliers
$131.00/250mg
Yield: 96%
Reaction Conditions:
with trichlorophosphate for 3 h;Reflux;
Steps:
Procedure for the synthesis of 2-chloro-7-nitroquinoxaline(4)
In a round bottom flask, a mixture of 7-nitroquinoxalin-2-ol (3) (12.73 g, 67 mmol) in 20 mL of phosphoryl chloride(POCl3) was refluxed for 3 h. Then, the mixture wasslowly poured into a stirred mixture of ice and water. Theprecipitate was filtrated and washed with water to obtain13.43 g of a red/cherry solid. Yield 96%; mp 183-186 °C(lit. 185-189 °C);5 ATR/FTIR ν / cm-1 3092, 3048, 1682,1525, 1350, 740; 1H NMR (200 MHz, DMSO-d6) δ 9.22(s, 1H, H3), 8.88 (d, 1H, J 3.0, H8), 8.59 (dd, 1H, J 2.0, 9.0, H6), 8.39 (d, 1H, J 9.0, H5); 13C NMR (100 MHz,DMSO-d6) δ 149.2, 148.8, 148.3, 142.8, 140.2, 130.9,124.1, 123.9; DEPT-135 (100 MHz, DMSO-d6) δ 148.8,130.9, 124.1, 123.9; MS 209.1 + 211.1.
References:
Do Amaral, Daniel N.;De Sá Alves, Fernando R.;Barreiro, Eliezer J.;Laufer, Stefan A.;Lima, Lídia M. [Journal of the Brazilian Chemical Society,2017,vol. 28,# 10,p. 1874 - 1878]
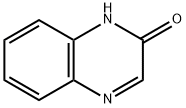
1196-57-2
332 suppliers
$6.00/10g

55686-94-7
64 suppliers
$131.00/250mg

109541-16-4
0 suppliers
inquiry

55686-94-7
64 suppliers
$131.00/250mg

6639-87-8
110 suppliers
$15.00/100mg

55686-94-7
64 suppliers
$131.00/250mg

95-54-5
542 suppliers
$9.00/1g

55686-94-7
64 suppliers
$131.00/250mg