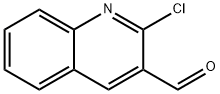
2-CHLOROQUINOLINE-3-CARBALDEHYDE synthesis
- Product Name:2-CHLOROQUINOLINE-3-CARBALDEHYDE
- CAS Number:73568-25-9
- Molecular formula:C10H6ClNO
- Molecular Weight:191.61

612-62-4

73568-25-9
Example 7 Synthesis of 2-chloro-3-quinolinecarboxaldehyde: 1.53 mL (3.30 mmol) of n-butyllithium (n-BuLi) was added slowly and dropwise to an 8 mL THF solution containing 0.46 mL (3.30 mmol) of diisopropylamine at 0 °C. After 20 minutes of reaction, the solution was cooled to -78 °C and 2-chloroquinoline (491 mg, 3.0 mmol) was added at this temperature. The mixture was stirred at -78 °C for 30 min, followed by dropwise addition of dimethylformamide (0.39 mL, 5.04 mmol) and continued stirring at the same temperature for 30 min. Upon completion of the reaction, the reaction was quenched with glacial acetic acid (1 mL) at -78 °C, then the mixture was slowly warmed to room temperature and diluted with ether (30 mL). The organic phase was washed sequentially with saturated NaHCO3 solution (10 mL) and brine (10 mL) and dried over anhydrous MgSO4. Concentration gave 2-chloro-3-quinolinecarboxaldehyde (530 mg, 92% yield) as a light yellow solid with melting point 145-149 °C, which could be used in the next reaction without further purification. By recrystallization from ethyl acetate, a pure product was obtained as pale yellow acicular crystals with a melting point of 149-150 °C (literature values 148-149 °C, see Meth-Cohn, O.; Narhe, B.; Tarnowski, B. J. Chem. Soc. Perkin Trans. I 1981, 1520).1H NMR (300 MHz, CDCl3) δ 10.57 (s, 1H), 8.77 (s, 1H), 8.08 (d, 1H, J = 9 Hz), 8.0 (d, 1H, J = 9 Hz), 7.90 (t, 1H, J = 9 Hz), 7.67 (t, 1H, J = 9 Hz); IR (nujol) 1685, 1575, 1045, 760, 745 cm-1. 745 cm-1.

612-62-4
272 suppliers
$35.00/5g

73568-25-9
193 suppliers
$9.00/250mg
Yield:73568-25-9 92%
Reaction Conditions:
with n-butyllithium;diisopropylamine in tetrahydrofuran;N-methyl-acetamide;acetic acid
Steps:
7 2-Chloro-3-quinolinecarboxaldehyde
EXAMPLE 7 2-Chloro-3-quinolinecarboxaldehyde To a solution of 0.46 mL (3.30 mmol) of diisopropylamine in 8 mL of THF at 0° C. was added 1.53 mL (3.30 mmol) of n-BuLi dropwise. After 20 min the solution was cooled to -78° C. and 2-chloroquinoline (491 mg, 3.0 mmol) was added neat. The mixture was stirred at -78° C. for 30 min, then dimethylformamide (0.39 mL, 5.04 mmol) was added dropwise and the reaction mixture was stirred an additional 30 min at this temperature. After quenching at -78° C. with glacial acetic acid (1 mL), the mixture was warmed to room temperature and diluted with ether (30 mL). The organic phase was washed with saturated NaHCO3 solution (10 mL) and brine (10 mL), and was dried over MgSO4. Concentration afforded 2-chloro-3-quinolinecarboxaldehyde (530 mg, 92%) as a light yellow solid (mp 145°-149 ° C.), which was used directly in the next step without further purification. Recrystallization from ethyl acetate afforded the pure compound as light yellow needles: mp 149°-150° C. (mp 148°-149° C. reported in Meth-Cohn, O.; Narhe, B.; Tarnowski, B. J. Chem. Soc. Perkin Trans. I 1981, 1520.). 1 H NMR (300 MHz, CDCl3) δ 10.57 (s, 1H), 8.77 (s, 1H), 8.08 (d, 1H, J=9 Hz), 8.0 (d, 1H, J=9 Hz), 7.90 (t, 1H, J=9 Hz), 7.67 (t, 1H, J=9 Hz); IR (nujol) 1685, 1575, 1045, 760, 745 cm-1.
References:
North Carolina State University US5212317, 1993, A
103-84-4
358 suppliers
$12.00/100g

73568-25-9
193 suppliers
$9.00/250mg

93299-49-1
36 suppliers
$244.00/500mg

73568-25-9
193 suppliers
$9.00/250mg
62-53-3
689 suppliers
$10.00/1g

73568-25-9
193 suppliers
$9.00/250mg

125917-60-4
52 suppliers
$60.00/100mg

1972-28-7
390 suppliers
$15.00/5g

73568-25-9
193 suppliers
$9.00/250mg
4114-28-7
74 suppliers
$70.50/25g