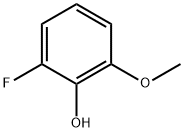
2-FLUORO-6-METHOXYPHENOL synthesis
- Product Name:2-FLUORO-6-METHOXYPHENOL
- CAS Number:73943-41-6
- Molecular formula:C7H7FO2
- Molecular Weight:142.13

456-49-5

73943-41-6
The general procedure for the synthesis of 2-fluoro-6-methoxyphenol from m-fluoroanisole was as follows: 4.0 mL (9.4 mmol) of 2.36 M n-butyllithium solution was slowly added dropwise over a period of 1 h to 10.0 mL of anhydrous tetrahydrofuran (THF) solution containing 1.28 g (10 mmol) of m-fluoroanisole (1) according to the method of Ladd and Weinstock The reaction temperature was maintained below -65 °C and cooled using a dry ice/acetone bath. The reaction mixture was stirred at -78 °C and under nitrogen protection for 2 hours. Subsequently, 1.17 g (11 mmol) of trimethoxyborane dissolved in 2.00 mL of anhydrous THF was added dropwise over 1 h at -78 °C and stirring was continued at -78 °C for 30 min. The reaction mixture was slowly warmed to 0 °C and 0.88 mL (15 mmol) of glacial acetic acid was added, followed by the dropwise addition of 1 mL (10 mmol) of 30% hydrogen peroxide (H2O2) solution. The reaction mixture was stirred at 25 °C overnight. After completion of the reaction, the reaction solution was diluted with 10 mL of water and extracted with THF (2 × 10 mL). The organic phases were combined and washed sequentially with H2O (2 × 7 mL) and 10% brine (2 × 3 mL). The organic phase was dried with anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure to give 1.16 g (80% yield) of 2-fluoro-6-methoxyphenol. The yields of successive replicate experiments were as follows: 15.5 g of product (63% yield) was obtained from 174 mmol of feedstock; 29.7 g of product (80% yield) was obtained from 261 mmol of feedstock. The structures of the products were analyzed by 1H NMR (300 MHz, CDCl3) δ 6.82-6.63 (m, 1H), 5.51 (s, 1H), 3.90 (s, 1H); 19F NMR (282 MHz, CDCl3) δ -137.61 ppm (m, 1F); EI-MS: m/z 142 (M+), 127.

456-49-5
371 suppliers
$8.00/10g

73943-41-6
101 suppliers
$13.00/250mg
Yield: 80%
Reaction Conditions:
Stage #1:1-fluoro-3-methoxybenzene with n-butyllithium in tetrahydrofuran at -78 - -65; for 2 h;
Stage #2: with Trimethyl borate in tetrahydrofuran at -78; for 1.5 h;
Stage #3: with dihydrogen peroxide;acetic acid in tetrahydrofuran at 0;
Steps:
Following the procedure of Ladd and Weinstock,5 4.0 mL (9.4 mmol) of 2.36 M n-butyl lithium wasadded dropwise over 1 hr to a solution of 1.28 g (10 mmol) fluoroanisole (1) in 10.0 mL dry THF keptbelow -65 oC by a dry ice/acetone bath. The solution was stirred for 2 hrs at -78 oC under nitrogen. 1.17g (11 mmol) of trimethoxyborane in 2.00 mL dry THF was added dropwise over 1 hr at -78 oC and thesolution was stirred for an additional 30 mins at -78 °C. The solution was warmed to 0 oC and 0.88 mL(15 mmol) of glacial acetic acid was added followed by the dropwise addition of 1 mL (10 mmol) of 30%H2O2. The solution was stirred overnight at 25 °C. The solution was diluted with H2O (10 mL) andextracted with THF (2 X 10 mL). The combined ether extracts were washed with H2O (2 X 7 mL) and 10%Mohrs salt (2 X 3 mL). The ether phase was dried with MgSO4 and evaporated under reduced pressureto yield 1.16 g of (80%) 2-fluoro-6-methoxyphenol. Yields for successive repetitions: 174 mmol gave 15.5g (63%); 261 mmol gave 29.7 g (80%). 1H NMR (300 MHz, CDCl3) 6.82 - 6.63 (m, 1H), 5.51 (s, 1H), 3.90(s, 1H). 19F NMR (282 MHz, CDCl3) -137.61 ppm (m, 1F). EI-MS: m/z 142 (M+), 127.
References:
Maresh, Justin J.;Ralko, Arthur A.;Speltz, Tom E.;Burke, James L.;Murphy, Casey M.;Gaskell, Zachary;Girel, Joann K.;Terranova, Erin;Richtscheidt, Conrad;Krzeszowiec, Mark [Synlett,2014,vol. 25,# 20,art. no. ST-2014-S0514-1,p. 2891 - 2894] Location in patent:supporting information
914454-79-8
3 suppliers
inquiry

73943-41-6
101 suppliers
$13.00/250mg

148872-80-4
4 suppliers
inquiry

73943-41-6
101 suppliers
$13.00/250mg

394-64-9
85 suppliers
$30.00/250mg

73943-41-6
101 suppliers
$13.00/250mg
96994-70-6
34 suppliers
$75.00/100mg

443-81-2
171 suppliers
$18.00/1g

73943-41-6
101 suppliers
$13.00/250mg