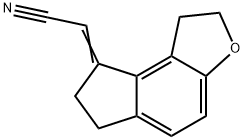
(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile synthesis
- Product Name:(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile
- CAS Number:221530-44-5
- Molecular formula:C13H11NO
- Molecular Weight:197.23
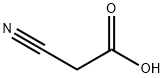
372-09-8
375 suppliers
$24.73/10gm:
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
262 suppliers
$31.00/250mg
![(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile](/CAS/GIF/221530-44-5.gif)
221530-44-5
23 suppliers
inquiry
Yield:221530-44-5 93%
Reaction Conditions:
with piperidine at 100 - 110;
Steps:
1
Example 1; Synthesis of (1, 6,7,8-Tetrahydro-2H-indeno-[5,4-b]furan-8-ylidene) -acetonitrile; l,2,6,7-Tetrahydro-8H-indeno-[5,4-b]furan-8-one (100 gm, 0.5747 mol ) and cyano acetic acid ( 75 gm, 0.8823 mol ) were added in piperidine (400 ml ) at ambient temperature. The reaction mass was heated to 100-110°C and then cooled to 55°C and the piperidine was removed under reduced pressure below 55°C. Water (500 ml) was added to the residue and extracted with dichloromethane (3 X 500 ml). Organic layer was washed with brine and distilled under reduced pressure below 50°C. The solid was stirred in heptane (500 ml), filtered, washed with heptane and dried under vacuum at 50-60°C to give 106 gms of the title compound.Yield:- 93%
References:
WO2012/35303,2012,A2 Location in patent:Page/Page column 23

2537-48-6
344 suppliers
$7.00/10g
![1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one](/CAS/GIF/196597-78-1.gif)
196597-78-1
262 suppliers
$31.00/250mg
![(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile](/CAS/GIF/221530-44-5.gif)
221530-44-5
23 suppliers
inquiry
![Acetonitrile, 2-[6-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2,3-dihydro-7-[2-[(methylsulfonyl)oxy]ethyl]-1H-inden-1-ylidene]-](/CAS/20210305/GIF/1242672-44-1.gif)
1242672-44-1
0 suppliers
inquiry
![(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile](/CAS/GIF/221530-44-5.gif)
221530-44-5
23 suppliers
inquiry
496-16-2
382 suppliers
$7.00/5g
![(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile](/CAS/GIF/221530-44-5.gif)
221530-44-5
23 suppliers
inquiry
![2,3-Dihydrobenzo[b]furan-5-carbaldehyde](/CAS/GIF/55745-70-5.gif)
55745-70-5
254 suppliers
$6.00/1g
![(1,2,6,7,-Tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile](/CAS/GIF/221530-44-5.gif)
221530-44-5
23 suppliers
inquiry