
3,4-dihydro-1-methyl-2(1H)-Quinoxalinone synthesis
- Product Name:3,4-dihydro-1-methyl-2(1H)-Quinoxalinone
- CAS Number:20934-50-3
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
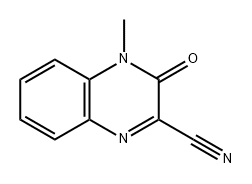
90800-73-0
0 suppliers
inquiry

20934-50-3
27 suppliers
inquiry
Yield:20934-50-3 75%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran at 50; for 1 h;
Steps:
1-Methyl-3,4-dihydroquinoxalin-2(1H)-one 1
To a stirred suspension of cyanoacetic acid (2.2 g, 26 mmol) in CH2Cl2 (100 mL) at 0 °C was added oxalyl chloride (2.0 mL, 24 mmol), followed by N,N-dimethylformamide (12 drops) and the mixture was warmed to room temperature (rt). After 3 h, N-methylaniline (2.2 mL, 20 mmol) in CH2Cl2 (80 mL) was added, followed by dropwise addition of trimethylamine (7.0 mL, 70 mmol) at 0 °C. The mixture was warmed to rt and stirred for 17 h. Water (40 mL) was added and the organic layer was separated, was washed with aqueous HCl (40 mL, 1 M), and was dried (MgSO4), filtered and concentrated under reduced pressure. Purification by column chromatography on silica gel,eluting with petrol-EtOAc (1:1), gave the amide A (3.4 g, 98%) as an amorphous solid; m.p. 74-76 °C (lit.1 76-79 °C); 1H NMR (400 MHz, CDCl3) δ = 7.58-7.39 (3H, m, 3 × CH), 7.33-7.20 (2H, m, 2 × CH), 3.34 (3H, s, CH3), 3.25 (2H, s, CH2); 13C NMR (100 MHz, CDCl3) δ = 161.7, 142.4, 130.5, 129.1, 127.1, 114.1, 37.9, 25.4. Data consistent with the literature.1 To a stirred solution of amide A (1.0 g, 5.7 mmol) in MeCN (29 mL) was added tert-butyl nitrite (3.5 mL, 29 mmol), Cs2CO3 (3.7 g, 12 mmol), glacial acetic acid (1.6 mL, 29 mmol) and 4 ? MS (2.9 g). The mixture was stirred at 100 °C for 6 h. Na2S2O4 (3.5 g, 20 mmol) in a mixture of EtOH (57 mL) and water (115 mL) was added and the mixture was heated for a further 2 h at 90 °C. After cooling the mixture to rt the mixture was extracted with EtOAc (2 × 250 mL), was washed with aq NaHCO3 (150 mL) and brine (150 mL), dried (MgSO4), filtered and concentrated under reduced pressure. The crude product was flushed through a silica gel plug, eluting with petrol-EtOAc (3:2) to give crude nitrile B (0.8 g, 74%) as an amorphous solid. To a solution of crude nitrile B (567 mg, 3.06 mmol) in dry THF (12 mL) was added NaBH4 (579 mg, 15.3 mmol). The mixture was heated at 50 °C for 1 h. After cooling to rt the mixture was diluted with water (50 mL) and EtOAc (150 mL). The organic layer was separated, was washed with brine (50 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. Purification by column chromatography on silica gel, eluting with petrol-EtOAc (1:1), gave the amine 1 (370 mg, 75%) which crystallised on standing; m.p. 52-54 °C; 1H NMR (400 MHz, DMSO-d6) δ = 6.96 (1H, d, J = 8.5 Hz, CH), 6.90-6.81 (1H, m, CH), 6.78-6.68 (2H, m, 2 × CH), 6.05 (1H, br s, NH), 3.77 (2H, s, CH2), 3.23 (3H, s, CH3); 13C NMR (100 MHz, DMSO-d6) δ = 165.8, 137.1, 129.0, 123.6, 118.6, 115.1, 114.0, 47.2, 28.6.
References:
Choi, Anthony;Coldham, Iain [Tetrahedron Letters,2019,vol. 60,# 37,art. no. 151023] Location in patent:supporting information

39581-30-1
9 suppliers
inquiry

20934-50-3
27 suppliers
inquiry