
3,5-Difluoro-4-pyridinecarboxaldehyde synthesis
- Product Name:3,5-Difluoro-4-pyridinecarboxaldehyde
- CAS Number:870234-98-3
- Molecular formula:C6H3F2NO
- Molecular Weight:143.09

107-31-3

71902-33-5

870234-98-3
General procedure for the synthesis of 3,5-difluoropyridine-4-carbaldehyde from methyl formate and 3,5-difluoropyridine: Preparation of Example 10: N-[(3,5-difluoro-4-pyridinyl)methyl]-2-(trifluoromethyl)pyridine-3-carboxamide (Compound A126) Step 1: Synthesis of 3,5-difluoropyridine-4-carbaldehyde A 2M solution of LDA (47.792 mL, 95.58 mmol) was diluted with 50 mL of THF at 0°C and cooled to -78°C. Subsequently, a 100 mL THF solution of 3,5-difluoropyridine (7.886 mL, 86.89 mmol) was added dropwise, keeping the temperature lower than -70°C (the dropwise addition process was controlled to be completed within 20 min). The reaction mixture formed a yellow suspension. Stirring was continued at -78°C for 3 hours. Then, 25 mL of THF solution of methyl formate (10.8 mL, 173.79 mmol) was added and the reaction mixture was transformed into a light yellow solution and stirred at -75°C for 45 minutes. The reaction mixture was transferred by cannula to 100 mL of stirred saturated aqueous NaHCO3 solution, maintained at about 0°C. The solution was extracted with EtOAc, the organic phases were combined and washed with brine and dried with 1N HCl. The solvent was evaporated at 165 mbar, 30°C to give 36.7 g of yellow liquid crude product. The crude product was purified by fast chromatography (eluent: CH2Cl2) to give a light yellow oil (7.85 g), which was crystallized after standing. 1H-NMR (CDCl3) data: δ 10.4 (s, 1H), 8.57 (s, 2H).

107-31-3
359 suppliers
$16.00/25mL

71902-33-5
169 suppliers
$6.00/1g

870234-98-3
88 suppliers
$57.00/100mg
Yield:870234-98-3 81%
Reaction Conditions:
Stage #1:3,5-difluoropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70; for 0.5 h;Inert atmosphere;
Stage #2:Methyl formate in tetrahydrofuran;hexane at -70; for 1.5 h;
Steps:
113A 3,5-Difluoroisonicotinaldehyde
Example 113A
3,5-Difluoroisonicotinaldehyde
Under argon and at -70° C., 44 ml of 2.5 M n-butyllithium solution in n-hexane (110 mmol, 1.1 equivalent) were slowly added dropwise to 15.4 ml of diisopropylamine (110 mmol, 1.1 equivalent) in 23 ml of THF.
The solution formed was warmed to 0° C. and stirred at this temperature for 30 min.
The reaction mixture was then brought to -70° C. and diluted with 23 ml of THF, and 11.5 g of 3,5-difluoropyridine (100 mmol, 1 equivalent) dissolved in 72 ml of THF, were added dropwise.
The mixture was stirred at -70° C. for 30 min. 12.4 ml of methyl formate (200 mmol, 2 equivalent), dissolved in 23 ml of THF, were then slowly added dropwise.
After 1.5 h at -70° C., the reaction solution was quickly poured into 230 ml of saturated aqueous sodium bicarbonate solution and extracted with a total of 460 ml of ethyl acetate.
The combined organic phases were washed twice with in each case 115 ml of saturated aqueous sodium bicarbonate solution and twice with saturated aqueous sodium chloride solution, dried over sodium sulphate and concentrated using a rotary evaporator.
This gave 11.6 g (81% of theory) of the title compound which were directly reacted further.
GC-MS (Method 14): Rt=1.82 min
MS (ESpos): m/z=144.0 (M+H)+
1H-NMR (400 MHz, DMSO-d6): δ=8.75 (br. s, 2H), 10.24 (br. s, 1H).
References:
BAYER PHARMA AKTIENGESELLSCHAFT;VAKALOPOULOS, Alexandros;HARTUNG, Ingo;FOLLMANN, Markus;JAUTELAT, Rolf;STRAUB, Alexander;HA?FELD, Jorma;LINDNER, Niels;SCHNEIDER, Dirk;WUNDER, Frank;STASCH, Johannes-Peter;REDLICH, Gorden;LI, Volkhart Min-Jian;BECKER-PELSTER, Eva Maria;KNORR, Andreas US2014/128386, 2014, A1 Location in patent:Paragraph 1410; 1411; 1412; 1413; 1414

71902-33-5
169 suppliers
$6.00/1g
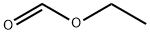
109-94-4
508 suppliers
$10.00/10g

870234-98-3
88 suppliers
$57.00/100mg

924649-13-8
8 suppliers
inquiry

870234-98-3
88 suppliers
$57.00/100mg

625-92-3
500 suppliers
$6.00/10g

870234-98-3
88 suppliers
$57.00/100mg
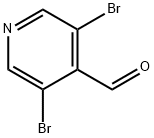
70201-42-2
212 suppliers
$5.00/1g

870234-98-3
88 suppliers
$57.00/100mg