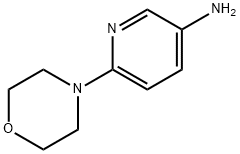
3-Amino-6-morpholinopyridine synthesis
- Product Name:3-Amino-6-morpholinopyridine
- CAS Number:52023-68-4
- Molecular formula:C9H13N3O
- Molecular Weight:179.22

26820-62-2

52023-68-4
General procedure for the synthesis of 3-amino-6-morpholinopyridine from 4-(5-nitropyridin-2-yl)morpholine: a mixture of 1.65 g (7.8 mmol) of 4-(5-nitro-2-pyridinyl)morpholine, 160 mg of 5% Pt/C catalyst, and 20 mL of ethanol was placed in a reactor. The reaction was carried out under 50 psi hydrogen atmosphere for 5 hours. Upon completion of the reaction, the mixture was filtered through a pad of diatomaceous earth to remove the catalyst, followed by evaporation of the solvent under reduced pressure to afford 1.4 g (100% yield) of 6-(4-morpholino)-3-pyridinamine, the product being a purple solid. Its 1H NMR (400 MHz, DMSO-d6) data were as follows: δ 7.60 (d, J=2.9 Hz, 1H), 6.92 (dd, J=8.8 and 2.9 Hz, 1H), 6.62 (d, J=8.8 Hz, 1H), 4.59 (brs, 2H), 3.65-3.72 (m, 4H), 3.17 (dt, J= 4.9 and 2.4 Hz, 4H).

110-91-8
709 suppliers
$9.00/1g

4548-45-2
548 suppliers
$6.00/5g

52023-68-4
131 suppliers
$45.00/50mg
Yield:52023-68-4 93%
Reaction Conditions:
Stage #1:morpholine;2-chloro-5-nitropyridine with triethylamine in dichloromethane at 20; for 12 h;
Stage #2: with palladium on activated charcoal;hydrogen in ethanol under 1125.11 Torr; for 2 h;
Steps:
3.1.3. Synthesis of 6-Morpholinopyridin-3-amine (6)
A mixture of 2-chloro-5-nitropyridine (303 mg, 1.91 mmol, 1.0 equiv), morpholine (0.5 mL,5.74 mmol, 3.0 equiv) and Et3N (483 mg, 0.67 mL, 2.5 equiv) in CH2Cl2 (4 mL) was stirred at roomtemperature overnight. The reaction mixture was diluted with water (10 mL) and extracted withCH2Cl2 (30 mL 3). The combined organic layers were washed with water (30 mL 6) and brine(1x30 mL), dried over anhydrous Na2SO4 and concentrated in vacuo to give a yellow solid. 70 mg ofthe yellow solid (0.335 mmol, 1.0 eq) were diluted in EtOH (5 mL) and a spatula tip of catalyst Pd/Cwas added. The obtained mixture was hydrogenated for 2 h, using a Hypem XP hydrogen generator(h2planet, Milan, Italy), Pressure was set at 1.5 bar. The crude mixture was filtered on Celite, and thefiltrate was evaporated to obtain a red solid. Yield: 93% over two steps. TLC (hexane:ethyl acetate =4:6 v/v + Et3N): Rf = 0.15. 1H-NMR (CDCl3) δ 7.79 (d, J = 2.7 Hz, 1H), 7.01 (dd, J = 8.8, 2.7 Hz, 1H),6.73 (brs, 2H, NH2), 6.56 (d, J = 8.8 Hz, 1H), 3.82 (m, 4H), 3.33 (m, 4H). 13C-NMR (CDCl3) δ 154.02,135.09, 134.58, 126.42, 108.41, 66.80 (2C), 47.08 (2C). ESI()MS: m/z 178 [M-H]-.
References:
Bosco, Bartolomeo;Defant, Andrea;Messina, Andrea;Incitti, Tania;Sighel, Denise;Bozza, Angela;Ciribilli, Yari;Inga, Alberto;Casarosa, Simona;Mancini, Ines [Molecules,2018,vol. 23,# 8,art. no. 1996]

26820-62-2
104 suppliers
$12.00/500mg

52023-68-4
131 suppliers
$45.00/50mg

110-91-8
709 suppliers
$9.00/1g

13534-97-9
437 suppliers
$6.00/1g

52023-68-4
131 suppliers
$45.00/50mg

4548-45-2
548 suppliers
$6.00/5g

52023-68-4
131 suppliers
$45.00/50mg

59027-83-7
10 suppliers
inquiry

52023-68-4
131 suppliers
$45.00/50mg