
3-IODOTHIOPHENE-2-CARBOXYLIC ACID synthesis
- Product Name:3-IODOTHIOPHENE-2-CARBOXYLIC ACID
- CAS Number:60166-84-9
- Molecular formula:C5H3IO2S
- Molecular Weight:254.05
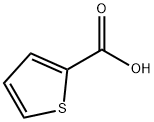
527-72-0

60166-84-9
Example 1 Preparation of 3-iodothiophene-2-carboxylic acid 1. dissolve thiophene-2-carboxylic acid (12.8 g, 0.1 mol) in THF at -78 °C. 2. n-Butyllithium (88 mL, 2.5 M THF solution, 2.2 eq.) was added slowly dropwise and stirring was continued for 0.5 hr after completion of the drop. 3. THF solution of iodine (28 g, 1.1 eq.) was added slowly with stirring. 4. The reaction mixture was gradually warmed to room temperature and stirred at room temperature until the reaction was complete. 5. The reaction mixture was concentrated to give a crude product. 6. The crude product was dissolved in EtOAc and extracted with 10% aqueous Na2CO3 solution. 7. The aqueous phases were combined and acidified with concentrated hydrochloric acid to pH<2. 8. The acidified aqueous phase was extracted with EtOAc and the organic phases were combined. 9. The organic phase was dried over anhydrous MgSO4, filtered and concentrated in vacuo to give 3-iodothiophene-2-carboxylic acid as a yellow-orange solid (23.5 g, 95% yield). 10. The product was characterized and confirmed by HPLC and mass spectrometry.

527-72-0
503 suppliers
$5.00/10g

60166-84-9
42 suppliers
$144.00/5g
Yield:60166-84-9 95%
Reaction Conditions:
Stage #1: 2-Thiophenecarboxylic acidwith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;
Stage #2: with iodine in tetrahydrofuran at -78 - 20;
Stage #3: with hydrogenchloride in water;
Steps:
1
EXAMPLE 1 Preparation of 2- (3-LODOTHIEN-2-VL) CARBOXVLIC acid A solution of 2-thiophenecarboxylic acid (12.8 g, 0.1 mol) in THF AT-78°C is treated dropwise with n-butyl lithium (88 mL of 2.5 M solution in THF, 2.2 equiv), stirred for 0.5 h, treated dropwise with a solution of iodine (28 g) in THF (1.1 equiv. ), allowed to warm to room temperature while stirring, and CONCENTRATED IN VACUO. The resultant residue is dissolved in EtOAc and extracted with 10% aqueous NA2C03. The aqueous extracts are combined, acidified with conc. HCI and extracted with EtOAc. The EtOAc extracts are combined, dried over MGS04 and concentrated in vacuo to afford the title product as a yellow-orange solid, 23.5 g (95% yield), identified by HPLC and mass spectral analyses.
References:
WO2005/12311,2005,A1 Location in patent:Page/Page column 22

62353-77-9
53 suppliers
$15.00/250mg

60166-84-9
42 suppliers
$144.00/5g

22288-78-4
394 suppliers
$11.00/5g

60166-84-9
42 suppliers
$144.00/5g
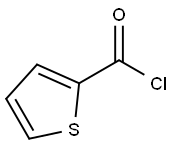
5271-67-0
259 suppliers
$5.00/1g

60166-84-9
42 suppliers
$144.00/5g

62521-42-0
2 suppliers
inquiry

60166-84-9
42 suppliers
$144.00/5g