
(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol synthesis
- Product Name:(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol
- CAS Number:302348-51-2
- Molecular formula:C13H19BO3
- Molecular Weight:234.1

128376-64-7
192 suppliers
$5.00/250mg

302348-51-2
134 suppliers
$5.00/250mg
Yield:302348-51-2 513 mg
Reaction Conditions:
with sodium tetrahydroborate at 20; for 5 h;
Steps:
[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]methanol (2a).
To a mixture of a4-formylbenzenboronic acid (1a, 375 mg, 2.50 mmol), pinacol (355 mg, 3.00 mmol) and anhydrous magnesium sulfate (625 mg, 5.00 mmol), methanol was added (12.50 mL). The mixture was stirred at room temperature for 6 h. After the reaction was completed, the crude solution was filtered, and then sodium borohydride (47 mg, 1.25 mmol) was added to the filtrate. Afterwards, the reaction mixture was stirred for an additional 5 h. Once the reaction was completed, the reaction mixture was filtered and the filtrate was concentrated in vacuo to give the desired product 2a as a white solid (m.p. 75-77 °C) in88% yield (513 mg). 1H-NMR (CD3OD-d4) δ ppm 7.71 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 7.8 Hz, 2H),4.62 (s, 2H), 1.34 (s, 12H); 13C-NMR (CD3OD-d4) δ ppm 146.23, 135.93, 127.26, 85.19, 65.24, 25.34;11B-NMR (CDCl3) δ ppm 34.82.
References:
Chung, Sheng-Hsuan;Lin, Ting-Ju;Hu, Qian-Yu;Tsai, Chia-Hua;Pan, Po-Shen [Molecules,2013,vol. 18,# 10,p. 12346 - 12367]

873-75-6
316 suppliers
$6.00/5g
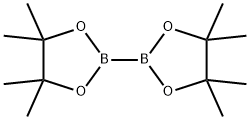
73183-34-3
590 suppliers
$6.00/5g

302348-51-2
134 suppliers
$5.00/250mg
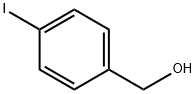
18282-51-4
217 suppliers
$10.00/1g

73183-34-3
590 suppliers
$6.00/5g

302348-51-2
134 suppliers
$5.00/250mg
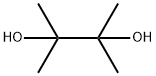
76-09-5
392 suppliers
$8.19/250mg

59016-93-2
327 suppliers
$5.00/1g

302348-51-2
134 suppliers
$5.00/250mg
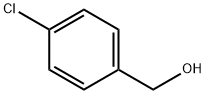
873-76-7
350 suppliers
$6.00/10g

73183-34-3
590 suppliers
$6.00/5g

302348-51-2
134 suppliers
$5.00/250mg