
(6-BROMO-2-PYRIDINYL)-CARBAMIC ACID,1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:(6-BROMO-2-PYRIDINYL)-CARBAMIC ACID,1,1-DIMETHYLETHYL ESTER
- CAS Number:344331-90-4
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13

19798-81-3

24424-99-5

344331-90-4
Step 1. Synthesis of 6-bromo-2-Boc-aminopyridine: To a solution of dichloromethane (24 mL) containing 6-bromopyridin-2-amine (3 g, 17.34 mmol), triethylamine (3.14 mL, 22.54 mmol), and DMAP (0.424 g, 3.47 mmol) was slowly added a solution of dichloromethane (6 mL) containing di-tert-butyl dicarbonate (4.83 mL, 20.81 mmol). 20.81 mmol) to a solution of dichloromethane (6 mL). The reaction mixture was stirred at room temperature for about 24 hours. Upon completion of the reaction, the reaction mixture was diluted with water, saturated saline and ethyl acetate. The aqueous layer was separated and extracted with ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography to give 6-bromo-2-tert-butoxycarbonylaminopyridine as a white solid. Yield: 1.67 g. LCMS (m/z): 274.9 [M+H]+; Retention time: 0.54 min (major peak), 0.95 min.

19798-81-3
447 suppliers
$5.00/1g

24424-99-5
859 suppliers
$13.50/25G

344331-90-4
104 suppliers
$24.00/100mg
Yield: 97%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 72 h;Product distribution / selectivity;
Steps:
Step 1. Preparation of te/f-butyl 6-bromopyridin-2-ylcarbamateTo a solution of 6-bromopyridin-2-amine (3 g, 17.34 mmol), triethylamine (3.14 mL, 22.54 mmol) and DMAP (0.424 g, 3.47 mmol) in DCM (24 mL) was added slowly a solution of BOC-anhydride (4.83 mL, 20.81 mmol) in DCM (6 mL). The reaction mixture was stirred at ambient temperature for -24 hr. The mixture was diluted with water, brine and EtOAc. The separated aqueous layer was extracted with EtOAc. The combined organic layers were dried over sodium sulfate and concentrated in vacuo. The resulting residue was purified by column chromatography providing te/f-butyl 6-bromopyridin-2- ylcarbamate as a white solid. Yield: 1.67 g. LCMS (m/z): 274.9 [M+H]+; Retention time = 0.95 min.
References:
NOVARTIS AG;ANTONIOS-MCCREA, William R.;BARSANTI, Paul A.;HU, Cheng;JIN, Xianming;LIN, Xiaodong;MARTIN, Eric J.;PAN, Yue;PFISTER, Keith B.;RENHOWE, Paul A.;SENDZIK, Martin;SUTTON, James;WAN, Lifeng WO2012/101066, 2012, A1 Location in patent:Page/Page column 77

21190-87-4
395 suppliers
$10.00/5g
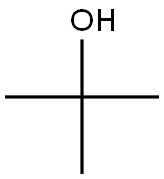
75-65-0
780 suppliers
$10.00/10ml

344331-90-4
104 suppliers
$24.00/100mg

19798-81-3
447 suppliers
$5.00/1g

75-44-5
0 suppliers
inquiry

75-65-0
780 suppliers
$10.00/10ml

344331-90-4
104 suppliers
$24.00/100mg

19798-81-3
447 suppliers
$5.00/1g

24424-99-5
859 suppliers
$13.50/25G

870703-61-0
30 suppliers
$45.95/1gm:

344331-90-4
104 suppliers
$24.00/100mg

19798-81-3
447 suppliers
$5.00/1g

34619-03-9
29 suppliers
inquiry

344331-90-4
104 suppliers
$24.00/100mg