
IMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:IMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:38922-77-9
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
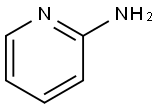
504-29-0
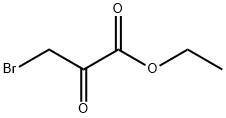
70-23-5
![IMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/38922-77-9.gif)
38922-77-9
Step 1: Synthesis of ethyl imidazo[1,2-a]pyridine-2-carboxylate Under magnetic stirring, 3 g (31.9 mmol) of 2-aminopyridine was dissolved in 65 mL of tetrahydrofuran. 4.44 mL (31.9 mmol) of ethyl 3-bromopyruvate was added slowly and solid precipitate formation was observed. The resulting non-homogeneous mixture was heated to reflux and stirred continuously for 4 hours. Upon completion of the reaction, it was cooled to room temperature and the solid product was isolated by filtration. The solid was dissolved in 65 mL of ethanol and again heated to reflux and stirred for 16 hours. The reaction mixture was cooled to room temperature and the solids were separated by filtration. The filtrate was placed in an ice bath to promote crystallization of the product until the liquid phase remained clear. The crystallized product was collected by filtration and washed with 30 mL of diisopropyl ether. Finally, the product was dried in a vacuum desiccator to afford 5.14 g (84% yield) of ethyl imidazo[1,2-a]pyridine-2-carboxylate as a white powder. Product characterization: LC-MS showed m/z=191 (MH+); UV purity was 99% at 254 nm.1H NMR (300 MHz, DMSO) δ 8.95 (s, 1H), 8.84 (d, J=6.8 Hz, 1H), 7.98-7.80 (m, 2H), 7.46 (t, J=7.2 Hz, 1H), 4.43 ( q, J=7.1Hz, 2H), 1.35 (d, J=7.1Hz, 3H).
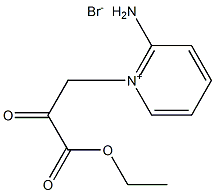
832099-51-1
2 suppliers
inquiry
![IMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/38922-77-9.gif)
38922-77-9
163 suppliers
$12.00/1g
Yield:-
Reaction Conditions:
in ethanol; for 3 h;Heating / reflux;
Steps:
20 Preparation 20, Ethyl imidazo[1,2-a]pyridine-2-carboxylate hydrobromide
A suspension of the compound from preparation 19 (71.9 g, 249 mmol) in ethanol (750 ml) was heated at reflux for 3 hours, then allowed to cool. The mixture was concentrated under reduced pressure, the residue triturated with ether, filtered and dried to afford the title compound as a solid, 64.17 g. 1H-NMR (CD3OD, 300 MHz) δ: 1.45 (t, 3H), 4.50 (q, 2H), 7.55 (m, 1H), 7.95 (m, 1H), 8.10 (dd, 1H), 8.80 (s, 1H), 8.85 (d, 1H).
References:
US2005/20611,2005,A1 Location in patent:Page/Page column 27