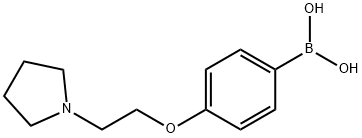
4-(2-(1-PYRROLIDINYL)ETHOXY)PHENYLBORON& synthesis
- Product Name:4-(2-(1-PYRROLIDINYL)ETHOXY)PHENYLBORON&
- CAS Number:226396-30-1
- Molecular formula:C12H18BNO3
- Molecular Weight:235.09
![N-[2-(4-Bromophenoxy)ethyl]pyrrolidine](/CAS/GIF/1081-73-8.gif)
1081-73-8
103 suppliers
$14.00/1g
5419-55-6
391 suppliers
$12.00/5g

226396-30-1
25 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-[2-(4-bromophenoxy)ethyl]pyrrolidinewith tert.-butyl lithium in tetrahydrofuran at -78; for 1.5 h;Cooling with acetone-dry ice;
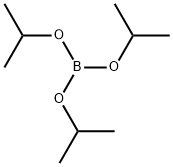
Stage #2: Triisopropyl borate in tetrahydrofuran at 20; for 15 h;
Stage #3: with hydrogenchloride in tetrahydrofuran at 20; for 2 h;
Steps:
16
Into a 16 x 100 mm vial was placed l-(2-(4-bromophenoxy)ethyl)pyrrolidine 60(100 mg, 0.370 mmol) and THF (2 mL). The vial was cooled to -78°C with a dry ice/acetonebath. To this was added tert-butyllithium (24 mg, 0.925 mmol) and the reaction was allowedto stir 1.5 hours. Triisopropylborate (84 mg, 0.444 mmol) was added dropwise and the reaction was allowed to reach room temperature and stirred for 15 hours.[0250] 6M HCl(aq) (0.2 mL) was added dropwise and allowed to stir for 2 hours at roomtemperature. The solvents were removed under reduced pressure, and the title compound 61was used without further purification.
References:
WO2006/4658,2006,A2 Location in patent:Page/Page column 52-53
![N-[2-(4-Bromophenoxy)ethyl]pyrrolidine](/CAS/GIF/1081-73-8.gif)
1081-73-8
103 suppliers
$14.00/1g

121-43-7
384 suppliers
$14.00/25mL

226396-30-1
25 suppliers
inquiry