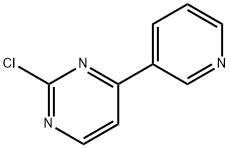
4-(3-Pyridyl)-2-chloropyrimidine synthesis
- Product Name:4-(3-Pyridyl)-2-chloropyrimidine
- CAS Number:483324-01-2
- Molecular formula:C9H6ClN3
- Molecular Weight:191.62

3934-20-1

1692-25-7

483324-01-2
In a 250 mL three-neck flask, 2,4-dichloropyrimidine (5.0 g, 33.56 mmol) was dissolved in a solvent mixture of 1,4-dioxane and water (4:1, 50 mL) and pyridine-3-boronic acid (4.95 g, 40.27 mmol), potassium carbonate (9.28 g, 67.12 mmol) and Pd(dppf)Cl2 (2.45 g , 3.36 mmol). The system was displaced three times with argon, gradually heated to 90 °C and reacted for 4 hours. Upon completion of the reaction, the system was concentrated under reduced pressure to remove most of the solvent, ethyl acetate (150 mL) and water (100 mL) were added for extraction and separation, and then the aqueous phase was extracted with ethyl acetate (80 mL). The organic phases were combined and washed with water (80 mL×2) and saturated saline (80 mL×2) sequentially, and the organic phase was dried with anhydrous sodium sulfate for 3 h. The crude product was obtained by filtration and concentration. The crude product was stirred with mixed solvents of petroleum ether and ethyl acetate (6 mL) for 1 h, filtered and dried in vacuum to obtain 4.8 g of 2-chloro-4-(3-pyridinyl)pyrimidine in 74.6% yield with HPLC purity >95%.

3934-20-1
725 suppliers
$9.00/1g

1692-25-7
484 suppliers
$5.00/1g

483324-01-2
89 suppliers
$65.00/10 mg
Yield: 81%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;caesium carbonate in tetrahydrofuran;water at 85; for 12 h;Inert atmosphere;
Steps:
Synthesis of 2-chloro-4-(pyridin-3-yl)pyrimidine (9)
A mixture of 2,4-dichloropyrimidine (7, 2.0 g, 13. 4 mmol), pyridin-3-ylboronic acid (8, 1.65 g 13.4 mmol), CS2CO3 (14.2 g, 40. 3 mmol) in THF (45 ml) and H2O (30 ml) was purged under Ar for about 10 minutes. PdCl2dppf.CH2Cl2 (650 mg, 0.81 mmol) was added to the reaction mixture and purged with Ar for another 5 minutes. The reaction mixture was then heated at 85 °C for 12 h. After completion of the reaction, the reaction mixture was cooled to room temperature and passed through a short Celite pad. The filtrate was partioned between EtOAc (60 ml) and H2O (30 ml). The aq layer was extracted with EtOAc (2 x 30 ml) and the combined organic layer was dried (Na2SO4) and concentrated, and the residue was purified on a Biotage Selekt flash purification system (silica gel, 3-5% MeOH/DCM), which afforded 2-chloro-4- (pyrid in-3-yl) pyrimid ine (9) as an off-white solid (2.08 g, 81%). 1H NMR (400 MHz, DMSO-d6) δ ppm 9.36 (d, J = 2.4 Hz, 1 H), 8.91 (d, J = 5.2 Hz, 1 H), 8.79 (dd, J = 4.8, 1 .6 Hz, 1 H), 8.56 (dt, J = 8.0, 2.4 Hz, 1 H), 8.27 (d, J = 5.2 Hz, 1 H), 7.63 (dd J = 8.0, 4.8 Hz, 1 H). ESI-MS m/z 192 [M + H]+. HPLC: Rt (min): 7.39 (Method 1).
References:
ESCO ASTER PTE. LTD.;CLEGG, Richard WO2021/74138, 2021, A1 Location in patent:Page/Page column 35

626-55-1
569 suppliers
$5.00/5 / G

1722-12-9
542 suppliers
$10.00/1g

483324-01-2
89 suppliers
$65.00/10 mg

897031-20-8
5 suppliers
inquiry

483324-01-2
89 suppliers
$65.00/10 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1722-12-9
542 suppliers
$10.00/1g

483324-01-2
89 suppliers
$65.00/10 mg

1722-12-9
542 suppliers
$10.00/1g

483324-01-2
89 suppliers
$65.00/10 mg