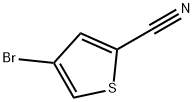
4-Bromothiophene-2-carbonitrile synthesis
- Product Name:4-Bromothiophene-2-carbonitrile
- CAS Number:18791-99-6
- Molecular formula:C5H2BrNS
- Molecular Weight:188.05

18791-75-8

18791-99-6
The general procedure for the synthesis of 4-bromothiophene-2-carbonitrile from 4-bromo-2-thiophenecarboxaldehyde was as follows: 4-bromothiophene-2-carboxaldehyde (600 g, 3.14 mmol) and hydroxylamine hydrochloride (438 g, 6.30 mmol) were dissolved in pyridine (5 L). After the reaction mixture was cooled to room temperature, it was heated to 90 °C and maintained for 10 min. Subsequently, acetic anhydride (1940 g, 19.0 mmol) was slowly added dropwise and the reaction system was heated to 80°C for 1 hour. After completion of the reaction, the mixture was poured into water (20 L) and stirred for 30 min. After filtration and drying, the target product 4-bromothiophene-2-carbonitrile (564 g, 95% yield) was obtained.

18791-75-8
285 suppliers
$9.00/5g

18791-99-6
96 suppliers
$14.00/1g
Yield: 95%
Reaction Conditions:
Stage #1:4-Bromothiophen-2-aldehyde with hydroxylamine hydrochloride in pyridine at 90; for 0.166667 h;
Stage #2: with acetic anhydride in pyridine at 80; for 1 h;
Steps:
3.3 (3) 4 - bromo thiophene -2 - carbonitrile synthesis
The 4 - bromo thiophene -2 - formaldehyde (600g, 3.14 μM) and hydroxylamine hydrochloride (438g, 6.30 μM) added to the pyridine (5L) in, heated to 90° C10 minutes after cooling down to room temperature, drop vinegar anhydride (1940g, 19.0 μM) heated to 80 °C, reaction 1 hours, poured into water to (20L) in, stirring 30 minutes, filtered, drying to obtain the product (564g, yield 95%).
References:
Shandong Xuanzhu Pharmaceutical Technology Co., Ltd.;Wu Yongqian CN104230960, 2017, B Location in patent:Paragraph 0449; 0450; 0451
83933-17-9
45 suppliers
$11.00/100mg

18791-99-6
96 suppliers
$14.00/1g
31767-01-8
14 suppliers
$156.00/1g

18791-99-6
96 suppliers
$14.00/1g