
(4-Hydroxy-2-methyl)phenylboronic acid synthesis
- Product Name:(4-Hydroxy-2-methyl)phenylboronic acid
- CAS Number:493035-82-8
- Molecular formula:C7H9BO3
- Molecular Weight:151.96
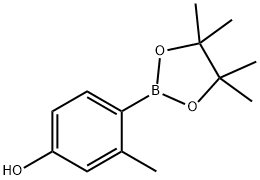
946427-03-8

493035-82-8
The general procedure for the synthesis of 4-hydroxy-2-methylphenylboronic acid from 3-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol is as follows: 1. sodium periodate (2.39 g, 11.15 mmol) was added to acetone and water (2:1, 36 mL) containing 3-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol (870.4 mg, 3.72 mmol) and ammonium acetate (859.8 mg, 11.15 mmol) in a room temperature in a mixed solution. 2. The reaction mixture was stirred for 68 hours, then filtered and concentrated. 3. 3. Sodium chloride was added to the concentrated filtrate and extracted with ethyl acetate. 4. 4. The organic layer was dried with anhydrous magnesium sulfate, followed by filtration and concentration. 5. 5. The residue was purified by silica gel chromatography using 1:4 ethyl acetate:hexane as eluent to afford 275.7 mg (49% yield) of 4-hydroxy-2-methylphenylboronic acid as a solid. Product characterization data: 1H NMR (400 MHz, d6-DMSO): δ 9.28 (s, 1H), 7.70 (d, J = 9 Hz, 1H), 6.56-6.52 (m, 2H), 2.55 (s, 3H). ESI-LCMS m/z 151 (M-H)-.

946427-03-8
46 suppliers
$110.00/500mg

493035-82-8
175 suppliers
$35.00/1g
Yield: 49%
Reaction Conditions:
with sodium periodate;ammonium acetate;water in acetone at 20; for 68 h;
Steps:
33.33b
33b) (4-hydroxy-2-methylphenyl)boronic acid Sodium periodate (2.39 g, 11.15 mmol) was added to 3-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol (870.4 mg, 3.72 mmol) and ammonium acetate (859.8 mg, 11.15 mmol) in acetone and water (2:1, 36 mL) at room temperature. The mixture was stirred for sixty-eight hours, then filtered, and concentrated. Sodium chloride was added to the filtrate, and it was extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, then filtered and concentrated. The residue was purified by silica gel chromatography eluding with 1:4 ethyl acetate:hexanes to give 275.7 mg (49%) of (4-hydroxy-2-methylphenyl)boronic acid as a solid. 1H NMR (400 MHz, d6-DMSO): δ 9.28 (s, 1H), 7.70 (d, J=9 Hz, 1H), 6.56-6.52 (m, 2H), 2.55 (s, 3H). ESI-LCMS m/z 151 (M-H)-.
References:
SmithKline Beecham Corporation US2008/96921, 2008, A1 Location in patent:Page/Page column 82