
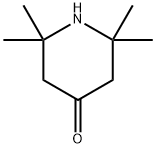
4-Oxo-TEMPO synthesis
- Product Name:4-Oxo-TEMPO
- CAS Number:2896-70-0
- Molecular formula:C9H16NO2*
- Molecular Weight:170.23
826-36-8
318 suppliers
$6.00/5g

2896-70-0
222 suppliers
$15.96/1G
Yield:2896-70-0 97%
Reaction Conditions:
with dihydrogen peroxide;Potassium bicarbonate in 5,5-dimethyl-1,3-cyclohexadiene;lithium hydroxide monohydrate;ethyl acetate at 40; pH=7.5; for 6 h;
Steps:
3 Method for preparing hindered amine nitroxide radical compound (2,2,6,6-tetramethylpiperidin-4-one nitroxide radical) by basic heterogeneous catalysis system
Take 2g (12mmol) of 2,2,6,6-tetramethylpiperidin-4-one (II-2) into a 50mL two-necked round bottom flask, install a thermometer sleeve, and add 10mL of reaction solvent to the reaction vessel (5mL xylene and 5mL ethyl acetate) dissolve the compound represented by formula II-2, maintain the reaction system at 40°C while continuously stirring, adjust the pH of the reaction system to 7.5 by using 0.01g/mL potassium bicarbonate aqueous solution, Slowly add 3.6mL 30% (mass concentration) hydrogen peroxide aqueous solution (36mmol) for reaction, TLC indicates the end point (about 6h), stop the reaction, separate the phases, collect the organic phase and dry it with anhydrous sodium sulfate, and concentrate the organic phase The target product 1.99 g of the compound of formula V-2 was obtained as a red solid with a yield of 97% and a purity of 99%.
References:
CN113429392,2021,A Location in patent:Paragraph 0046-0049; 0058-0059

33973-59-0
101 suppliers
$42.00/5g

2896-70-0
222 suppliers
$15.96/1G

3637-11-4
17 suppliers
inquiry

2896-70-0
222 suppliers
$15.96/1G

2403-88-5
346 suppliers
$5.00/10g

2226-96-2
512 suppliers
$5.00/5g

2896-70-0
222 suppliers
$15.96/1G