
4-Piperidinoaniline synthesis
- Product Name:4-Piperidinoaniline
- CAS Number:2359-60-6
- Molecular formula:C11H16N2
- Molecular Weight:176.26

6574-15-8
100 suppliers
$10.00/1g

2359-60-6
0 suppliers
$14.14/1gm:
Yield: 100%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethyl acetate at 20; for 3 h;
Steps:
2
4-Fluoronitrobenzene (323 mg, 2.3 mmol) was dissolved in DMSO (5 ml), potassium carbonate (475 mg, 3.5 mmol) and piperidine (460 μl, 4.6 mmol) were added, and the mixture was stirred at 90° C. for 9 hr.
Then, water was added to the reaction solution, and the mixture was extracted twice with ethyl acetate.
The organic layer was washed twice with saturated aqueous NaCl.
The organic layer was dried over Na2CO3, the solvent was evaporated to give compound Y197 (yield; 472 mg, 100%).
Compound Y197 was dissolved in ethyl acetate (20 ml), Pd/C (186 mg) was added, and the mixture was stirred under a hydrogen atmosphere at room temperature for 3 hr.
Then, the reaction solution was filtered through celite, the filtrate was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (40:1)) to give compound Y222 (yield, quantitative, 394 mg).
Compound Y491 (mentioned later) (80 mg, 0.18 mmol) was dissolved in dichloromethane (2 ml), compound Y222 (100 mg, 0.58 mmol) was added, and the mixture was stirred at room temperature for 5 hr.
Then, the solvent was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (35:1)) to give the title compound (yield; 68 mg, 64%).
1H NMR (500 MHz, CDCl3) δ8.40 (s, 1H), 8.0 (d, 1H, J=8.0 Hz), 7.70 (d, 1H, J=8.5 Hz), 7.52 (dd, 1H, J=8.0, 7.5 Hz), 6.92 (dd, 2H, J=9.0, 3.5 Hz), 6.77 (dd, 2H, J=9.0, 6.5 Hz), 5.33 (t, 1H, J=6.0 Hz), 4.07 (bs, 2H), 3.11-3.09 (m, 4H), 2.79-2.64 (m, 4H), 1.69-1.53 (m, 10H), 1.43 (s, 9H), 1.09-1.01 (m, 2H)
13C NMR (125 MHz, CDCl3) δ154.9, 141.6, 140.8, 131.4, 130.9, 129.8, 125.8, 125.7, 79.7, 77.4, 48.7, 36.6, 29.6, 28.6, 25.7, 24.2
HRMS (FAB-) m/z: [M-H]- calcd for C28H39N4O6S2, 591.2311. found, 591.2324
References:
KYOTO UNIVERSITY;Kakeya, Hideaki;Hattori, Akira;Takasu, Yasuaki;Fujii, Nobutaka;Oishi, Shinya;Kojima, Soichi;Hara, Mitsuko US2013/45977, 2013, A1 Location in patent:Paragraph 0211; 0212; 0213; 0214
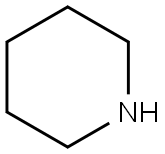
110-89-4
0 suppliers
$15.96/100ML

106-47-8
498 suppliers
$5.00/5 g

2359-60-6
0 suppliers
$14.14/1gm:

110-89-4
0 suppliers
$15.96/100ML

106-40-1
492 suppliers
$12.00/5g

2359-60-6
0 suppliers
$14.14/1gm:
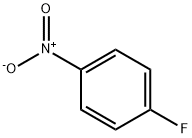
350-46-9
526 suppliers
$10.60/1gm:

2359-60-6
0 suppliers
$14.14/1gm:

110-89-4
0 suppliers
$15.96/100ML

2359-60-6
0 suppliers
$14.14/1gm: