
4-(Trifluoromethoxy)fluorobenzene synthesis
- Product Name:4-(Trifluoromethoxy)fluorobenzene
- CAS Number:352-67-0
- Molecular formula:C7H4F4O
- Molecular Weight:180.1

338792-64-6

352-67-0
The general procedure for the synthesis of p-fluorotrifluoromethoxybenzene from 2,2-difluoro-2-(4-fluorophenoxy)acetic acid was as follows: a stir bar was added to a 5 mL microwave vial in an argon-protected glove box and sealed. Selectfluor (177.1 mg, 0.5 mmol, 2.0 eq.), silver trifluoromethanesulfonate (12.8 mg, 0.05 mmol, 20 mol%), and 2,2-difluoro-2-(4-fluorophenoxy)acetic acid (0.25 mmol, 1.0 eq.) were added sequentially. The vial was charged with dichloromethane (1.8 mL), trifluoroacetic acid (76.5 μL, 1.0 mmol, 4.0 equiv) and water (0.2 mL). The reaction mixture was heated at 55 °C for 1 hour. After completion of the reaction, the mixture was cooled to room temperature, diluted with dichloromethane (4 mL), washed sequentially with water (3 x 5 mL) and brine (5 mL), dried over anhydrous magnesium sulfate and filtered. The dried organic phase was concentrated on a rotary evaporator. The crude product obtained was dissolved in a small amount of dichloromethane and purified by passing through a silica gel column (10 g, Biotage), first dried with air and then eluted with pentane. The fractions containing the pure product were combined and the solvent evaporated to give pure p-fluorotrifluoromethoxybenzene.

338792-64-6
1 suppliers
inquiry

352-67-0
209 suppliers
$7.00/5g
Yield:352-67-0 19 %Spectr.
Reaction Conditions:
with silver trifluoromethanesulfonate;Selectfluor;trifluoroacetic acid in dichloromethane;water at 55; for 1 h;Inert atmosphere;Glovebox;Reagent/catalyst;
Steps:
4.3. Fluorodecarboxylation aryloxydifluoroacetic acids
General procedure: A microwave vial (5 mL) was sealed under an inert atmosphere (Argon glovebox) with a stir bar, Selectfluor (177.1 mg, 0.5 mmol, 2 equiv), silver trifluoromethanesulfonate (12.8 mg, 0.05 mmol, 20 mol%) and aryloxydifluoroacetic acids (0.25 mmol, 1.0 equiv). To this vial DCM (1.8 mL), trifluoroacetic acid (76.5 μL, 1.0 mmol, 4.0 equiv) and water (0.2 mL) were injected. This mixture was heated for an hour at 55 °C. The resulting mixture was cooled down to room temperature, diluted with dichloromethane (4 mL), washed with water (3×5 mL), brine (5 mL), dried over anhydrous MgSO4 and filtered. The dried extract was concentrated on a rotary evaporator. The resulting crude product was dissolved in a small quantity of dichloromethane and loaded on to a silica cartridge (10 g, Biotage), airdried and eluted with pentane. The pure fractions were combined and the solvent was evaporated to obtain the pure products.
References:
Krishanmoorthy, Sankarganesh;Schnell, Simon D.;Dang, Huong;Fu, Fang;Prakash, G.K. Surya [Journal of Fluorine Chemistry,2017,vol. 203,p. 130 - 135]
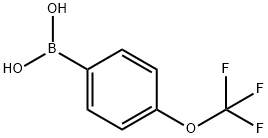
139301-27-2
385 suppliers
$14.00/5G

352-67-0
209 suppliers
$7.00/5g

371-41-5
528 suppliers
$8.00/25g

352-67-0
209 suppliers
$7.00/5g

807368-70-3
9 suppliers
inquiry

352-67-0
209 suppliers
$7.00/5g

