
5,7-DIMETHYLISATIN synthesis
- Product Name:5,7-DIMETHYLISATIN
- CAS Number:39603-24-2
- Molecular formula:C10H9NO2
- Molecular Weight:175.18

7343-12-6

39603-24-2
The general procedure for the synthesis of 5,7-dimethylindoline-2,3-dione using (E)-N-(2,4-dimethylphenyl)-2-(hydroxyimino)acetamide as starting material was as follows: concentrated sulfuric acid (20 mL) was added to a 100 mL flask with vigorous stirring at 50°C, and N-2-(hydroxyimino)acetamide derivatives (7.0 g) were added in batches. The reaction temperature was maintained between 50°C and 75°C, followed by the addition of sulfide. After the addition was completed, the reaction mixture was warmed to 80°C and stirring was continued for 30 minutes. After completion of the reaction, the mixture was cooled to room temperature and slowly poured into ice (250 g). The precipitated solid was collected and dried in air to give the crude product. The crude product was dissolved in 5% dilute sodium hydroxide solution (100 mL) and acidified with 4N hydrochloric acid (20 mL). The precipitated solid was filtered and dried thoroughly to give the purified target compound 5,7-dimethylindoline-2,3-dione.
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

39603-24-2
118 suppliers
$51.00/10g
Yield: 60%
Reaction Conditions:
with sulfuric acid at 80; for 0.666667 h;Cooling with ice;
Steps:
5-Hexyl-2,3-dihydro-1H-indole-2,3-dione (9)
General procedure: A round-bottom flask is charged with concentrated sulfuric acid (33 ml) and is magnetically stirred at 50 °C open to the atmosphere. To the acid was added 2-[(benzyloxy)imino]-N-(4-n-hexylphenyl) (11 g, 32.5 mmole) in portions over 30 minutes. On completion of the addition the mixture was heated at 80 °C for an additional 10 minutes then allowed to cool to room temperature. The dark viscous solution was subsequently added in portions with rapid stirring to ice (750 ml). The crude product was isolated by vacuum filtration, and the filter cake washed with water. The crude product was dried under vacuum to give 6.3 g (85 %) of an orange solid.
References:
Klein, Larry L.;Tufano, Michael D. [Tetrahedron Letters,2013,vol. 54,# 8,p. 1008 - 1011] Location in patent:supporting information

7343-12-6
10 suppliers
inquiry

39603-24-2
118 suppliers
$51.00/10g
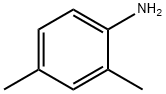
95-68-1
316 suppliers
$14.00/5g

39603-24-2
118 suppliers
$51.00/10g

4102-54-9
15 suppliers
$65.09/1g

39603-24-2
118 suppliers
$51.00/10g
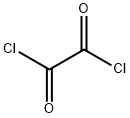
79-37-8
488 suppliers
$17.67/10gm:

95-68-1
316 suppliers
$14.00/5g

39603-24-2
118 suppliers
$51.00/10g