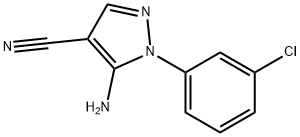
5-AMINO-1-(3-CHLOROPHENYL)-1H-PYRAZOLE-& synthesis
- Product Name:5-AMINO-1-(3-CHLOROPHENYL)-1H-PYRAZOLE-&
- CAS Number:51516-68-8
- Molecular formula:C10H7ClN4
- Molecular Weight:218.64

2312-23-4

123-06-8

51516-68-8
Sodium hydride (60% mineral oil dispersion, 0.48 g, 12 mmol, 1.2 eq.) was slowly added to anhydrous ethanol (20 ml) at 0 °C. To the resulting ethanol solution of sodium ethanol was sequentially added 3-chlorophenylhydrazine hydrochloride (1.79 g, 10 mmol, 1.0 eq.) and ethoxymethylene malononitrile (1.22 g, 10 mmol, 1.0 eq.). The reaction mixture was heated to reflux with continuous stirring for 1 hour. After completion of the reaction, the mixture was cooled to room temperature and precipitation was observed to be generated. Anhydrous ethanol (20 ml) was added to the reaction mixture for dilution, the precipitate was collected by filtration, washed with ether (2 x 100 ml) and dried under vacuum to afford the target compound 5-amino-1-(3-chlorophenyl)-1H-pyrazole-4-carbonitrile as a yellow solid (1.56 g, 7.13 mmol, 72% yield).LCMS analysis: [M + H]+ = 219, retention time Rt = 1.17min, purity 100%.

2312-23-4
224 suppliers
$7.00/10g

123-06-8
356 suppliers
$14.14/1gm:

51516-68-8
59 suppliers
$12.00/250mg
Yield:51516-68-8 72%
Reaction Conditions:
with potassium etoxide;sodium hydride in ethanol at 0; for 1 h;Heating / reflux;
Steps:
1.1a.1.2A
Sodium hydride as a 60% dispersion in mineral oil (0.48 g, 1.2 eq, 12 mmol) was added slowly to ethanol (20 ml) at 0°C. To the solution of sodium ethoxide in ethanol was added 3-chlorophenylhydrazine hydrochloride (1.79 g, 1.0 eq, 10 mmol), addition of ethoxymethylene malonitrile (1.22 g, 1.0 eq, 10 mmol) shortly followed. The reaction mixture was heated to reflux with stirring for 1 hour. The reaction mixture was then allowed to cool to room temperature, once at room temperature a precipitate was observed. Ethanol (20 ml) was added to the slurry and the precipitate was collected by filtration, washed with diethyl ether (2 x 100 ml) and dried in vacuo to give the title compound as a yellow solid (1.56 g, 7.13 mmol, 72%). LCMS: [M+H]+=219, Rt = 1.17 min, 100% purity.
References:
EP1746099,2007,A1 Location in patent:Page/Page column 14

123-06-8
356 suppliers
$14.14/1gm:

14763-20-3
23 suppliers
inquiry

51516-68-8
59 suppliers
$12.00/250mg

108-42-9
477 suppliers
$10.00/5g

51516-68-8
59 suppliers
$12.00/250mg
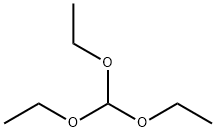
122-51-0
521 suppliers
$10.00/5ml

14763-20-3
23 suppliers
inquiry

109-77-3
453 suppliers
$10.00/5g

51516-68-8
59 suppliers
$12.00/250mg