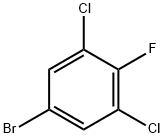
5-Bromo-1,3-dichloro-2-fluorobenzene synthesis
- Product Name:5-Bromo-1,3-dichloro-2-fluorobenzene
- CAS Number:17318-08-0
- Molecular formula:C6H2BrCl2F
- Molecular Weight:243.89

60811-21-4

17318-08-0
The general procedure for the synthesis of 3,5-dichloro-4-fluorobromobenzene from 3-chloro-4-fluorobromobenzene was as follows: to a dry Schlenk flask equipped with a magnetic stirrer and septum under argon protection was added 20 mL of freshly titrated iPrMgCl-LiCl (1.24 M, THF solution, 1.0 equiv.), followed by a slow dropwise addition of 3.8 mL of diisopropylamine (1.1 equiv.) ). The reaction mixture was stirred at room temperature until complete gas release (~48 hours). The precipitate formed was dissolved with additional dry THF. A fresh solution of iPr2NMgCl-LiCl in THF was titrated at 25 °C using benzoic acid and 4-(phenylazo)diphenylamine as indicators, and the concentration was measured to be 0.59 M. 4-Bromo-2-chloro-1-fluorobenzene (0.209 g, 1.00 mmol) was dissolved in THF (1 mL) and iPr2NMgCl-LiCl (0.59 M , 3.39 mL, 2.00 mmol) solution was added and stirred at 25 °C for 15 min. Subsequently, hexachloro-2-acetone (0.397 g, 1.50 mmol) was added at 0 °C and stirring was continued for 15 min. The reaction mixture was quenched with saturated aqueous NaHCO3 solution, extracted with ethyl acetate and the organic phase was dried over anhydrous Na2SO4. After filtration, the solvent was concentrated under reduced pressure. Quantitative GC analysis showed the ratio of 5-bromo-1,3-dichloro-2-fluorobenzene to the regional isomers to be about 12:1. Purification by fast column chromatography (silica gel, n-hexane) afforded 5-bromo-1,3-dichloro-2-fluorobenzene (0.190 g) as a colorless oil.

1517200-74-6
28 suppliers
inquiry

17318-08-0
184 suppliers
$6.00/1g
Yield:17318-08-0 83.1%
Reaction Conditions:
Stage #1: 5-bromo-3-chloro-2-fluoroanilinewith hydrogenchloride;sodium nitrite in water at 10;
Stage #2: with hydrogenchloride;copper(l) chloride in water at 50; for 5 h;
Steps:
3 Preparation of 3,5-dichloro-4-fluorobromobenzene
Put 224.5g (1mol) of 2-fluoro-3-chloro-5-bromoaniline, 608g (5mol) of hydrochloric acid and 300g of water into a 2L four-necked flask, cool to 10, and add 103.5g (1.5mol) of sodium nitrite dropwise. A solution of 200 g of water is dripped to obtain a diazonium liquid. Put 300g of hydrochloric acid and 100g of cuprous chloride into another 2L four-necked flask, raise the temperature to 50°C, add step diazonium solution dropwise, keep it at this temperature for 5h after dropping, control the sampling, the reaction is complete, cool to room temperature, and let it rest. The layers were separated, the oil layer was neutralized, washed with water, separated into layers, distilled, and then rectified to obtain 203 g of 3,5-dichloro-4-fluorobromobenzene with a purity of 99.8% and a yield of 83.1%.
References:
CN112010732,2020,A Location in patent:Paragraph 0047-0052

60811-21-4
226 suppliers
$9.00/1g

17318-08-0
184 suppliers
$6.00/1g
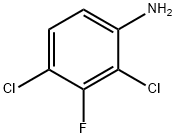
443-93-6
49 suppliers
$60.00/50mg

17318-08-0
184 suppliers
$6.00/1g
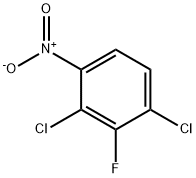
393-79-3
154 suppliers
inquiry

17318-08-0
184 suppliers
$6.00/1g

2268-05-5
233 suppliers
$5.00/5g

17318-08-0
184 suppliers
$6.00/1g