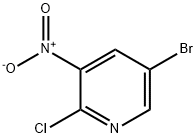
5-Bromo-2-chloro-3-nitropyridine synthesis
- Product Name:5-Bromo-2-chloro-3-nitropyridine
- CAS Number:67443-38-3
- Molecular formula:C5H2BrClN2O2
- Molecular Weight:237.44

6945-68-2

67443-38-3
The general procedure for the synthesis of 2-chloro-3-nitro-5-bromopyridine from 2-amino-3-nitro-5-bromopyridine was as follows: 2-amino-5-bromo-3-nitropyridine (21.8 g, 0.1 mol) was ground in a mortar and pestle to a fine powder, which was subsequently suspended in 6 M hydrochloric acid (250 ml). The mixture was cooled to 0 °C and solid NaNO2 (8.3 g, 0.12 mol) was slowly added while keeping the internal temperature below 5 °C (about 45 min). After addition, stirring was continued at 0 °C for 1 hour. To the resulting suspension was added a solution of freshly prepared copper(I) chloride (12.9 g, 0.13 mol) dissolved in degassed 38% hydrochloric acid, and the reaction mass was gradually warmed up to room temperature over 90 min, and then subsequently heated up to 70°C to complete the decomposition of the diazonium salt. The reaction mixture was cooled, diluted with water (750 ml), passed through air for 30 min, and the pH was subsequently adjusted by the addition of 0.88 ammonia to about 9. The blue mixture was mixed with ether (600 ml) and shaken, and the insoluble solids were removed by filtration. The organic layer was washed sequentially with 5% ammonia, water, and brine and dried with anhydrous sodium sulfate. After filtration, the solution was pre-adsorbed onto silica and purified by fast chromatography using a gradient elution of isohexane with 5% to 20% ethyl acetate to afford 5-bromo-2-chloro-3-nitropyridine as a light yellow solid (13.1 g, 55% yield) and to recover the starting material (6.4 g). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.36 (1H, d, J=1Hz, H-6), 8.69 (1H, d, J=1Hz, H-4).

6945-68-2
407 suppliers
$9.00/5g

67443-38-3
382 suppliers
$5.00/1g
Yield:67443-38-3 84%
Reaction Conditions:
with lithium chloride
Steps:
2
The λ/-[5-(5-amino-6-chloropyridin-3-yl)thiazol-2-yl]acetamide used as a starting material was prepared as follows :-Using an analogous procedure to that described by K. Jouve and J. Bergman, J. Heterocyclic Chem., 2003, 40, 261 except that one equivalent of lithium chloride was used, 2-amino-5-bromo-3-nitropyridine was converted in 84% yield into 5-bromo-2-chloro-3-nitropyridine; 1H νMR Spectrum: (CDCl3) 8.37 (IH, d), 8.7 (IH, d); which, in turn, was converted into 3-amino-5-bromo-2-chloropyridine; 1H νMR Spectrum: (CDCl3) 4.17 (2H, br s), 7.18 (IH, d), 7.85 (IH, d).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2007/129044, 2007, A1 Location in patent:Page/Page column 77
15862-34-7
291 suppliers
$10.00/5g

67443-38-3
382 suppliers
$5.00/1g

15862-34-7
291 suppliers
$10.00/5g

67443-38-3
382 suppliers
$5.00/1g

246543-32-8
0 suppliers
inquiry

67443-38-3
382 suppliers
$5.00/1g

15862-34-7
291 suppliers
$10.00/5g

7440-44-0
349 suppliers
$12.00/25g

67443-38-3
382 suppliers
$5.00/1g